Abstract
Aim: Short-term results of durable polymer everolimus-eluting stents have shown significant improvements in clinical and angiographic outcomes. This report presents the 1-year clinical and angiographic data from the SPIRIT FIRST Trial.
Methods and results: This first-in-man single blind multi-centre randomized controlled trial assessed the safety and efficacy of everolimus and a durable polymer on a cobalt chromium stent in patients with de novo native coronary artery lesions. Of the 60 patients enrolled, a total of 56 patients (27 everolimus arm and 29 bare stent arm) were qualified to per-treatment analyses at 1 year. Quantitative angiographic and intravascular ultrasound (IVUS) analyses were performed. Angiographic late loss, IVUS neointimal volume obstruction and major adverse cardiac events (MACE) at 1 year were assessed as the study endpoints. At 1 year, the in-stent late loss and diameter stenosis of patients were 0.24 mm and 18% in the everolimus arm (n=20), as compared with 0.84 mm and 37% in the bare stent arm (n=25, p < 0.001). Significantly less neointimal hyperplasia was observed in the everolimus arm compared to the bare stent arm (neointimal volume, 13±9 mm3 vs. 37±17 mm3, p < 0.001; volume obstruction, 10±7% vs. 28±12%, p < 0.001). The overall MACE rate was 15.4% in the everolimus arm and 21.4% in the bare stent arm.
Conclusion: The safety and efficacy of everolimus-eluting stent with a durable polymer observed at 6 months was sustained at 1 year.
Introduction
To date percutaneous coronary intervention (PCI) using drug-eluting stents is considered the most secure treatment option for de novo single coronary artery disease. The two clinically available stents coated with an anti-proliferative drug, sirolimus or paclitaxel, have shown promising clinical and angiographic outcomes as proven in several randomized trials1-3. Beside these two drugs, the efficacy of newly developed antiproliferative drugs has been clinically investigated4-9 and their potent effects in preventing restenosis have been reported5-9.
Everolimus is a powerful anti-proliferative agent and has shown effect in preventing rejection in kidney and heart transplantation10-12. In the presence of everolimus, the growth factor-stimulated phosphorylation of p70 S6 kinase and 4E-BP1 is inhibited. The latter proteins are key proteins involved in the initiation of DNA synthesis. Since phosphorylation of both p70 S6 kinase and 4E-BP1 is under the control of FRAP (FKBP-12-rapamycin associated protein, also called mTOR, mammalian Target Of Rapamycin), this finding suggests that, like sirolimus, the everolimus-FKBP12 complex binds to and thus interferes with the function of FRAP.
The SPIRIT FIRST clinical trial represents the first evaluation of the everolimus-eluting stent which studied the potential benefits of the local application of everolimus in a durable polymer in combination with a stent with a thin strut design5. Compared to identical bare metal stents, everolimus-eluting stents have demonstrated effective suppression of neointimal growth at 6 months5. This paper presents the 1-year clinical and angiographic/intravascular ultrasound (IVUS) follow-up results from the experience with the durable polymer everolimus-eluting stent.
Methods
Study population
The SPIRIT FIRST clinical trial was a prospective, controlled, randomized, single-blinded, parallel 2-arm, multicentre clinical evaluation of a durable polymer everolimus-eluting stent (XIENCE™ V, Guidant, Santa Clara, CA, USA) in patients with de novo native coronary artery lesions. Patient eligibility criteria, device description and study procedure were previously reported, along with 6-month clinical, angiographic and IVUS analyses5. Briefly, study patients had single de novo stenoses of < 18 mm lesion length, coverable by 1 study stent, > 50% diameter stenosis, and vessel reference diameter 3.0 mm as assessed by on-line quantitative coronary angiography (QCA). Patients were ineligible if they had any of the followings: evolving myocardial infarction; stenosis of an unprotected left main coronary artery, an ostial location, or located within 2 mm of a bifurcation; a lesion with moderate to heavy calcification, or an angiographically visible thrombus; a left ventricular ejection fraction < 30%; were awaiting a heart transplant, or had a contraindication to aspirin, clopidogrel, heparin and any other drugs related to this study.
Follow-up and study endpoint
Clinical evaluation was scheduled at 1, 6, and 12 months with annual evaluation up to 5 years. Angiographic and IVUS imaging was obtained at baseline, 6- and 12-month follow-up.
The primary endpoint was in-stent late loss at 6 months. The major secondary endpoint was percent (%) in-stent volume obstruction at 6 months based on IVUS analysis. Other secondary endpoints included the followings: a) in-stent late loss at 1 year; b) in-segment late loss at 6 months and 1 year including proximal and distal evaluations; c) in-stent% volume obstruction at 1 year; d) in-stent and in-segment% diameter stenosis at 6 months and 1 year; e) in-stent and in-segment angiographic binary restenosis (ABR) at 6 months and 1 year; f) persisting incomplete apposition, late incomplete apposition, aneurysm formation, thrombus, persisting dissection at 6 months and 1 year; g) major adverse cardiac events (MACE) rate in-hospital and at 1, 6, 9 months and annually up to 5 years. MACE is comprised of death, myocardial infarction (MI), or clinically driven target lesion revascularization (TLR); g) acute device, procedural and clinical success. All deaths that could not be clearly attributed to another cause were considered a cardiac death. A non-Q-wave myocardial infarction was defined by an increase in the creatine kinase level to more than twice the upper limit of the normal range, accompanied by an increased level of creatine kinase MB, in the absence of new Q waves on the surface electrocardiogram.
Quantitative Coronary Angiography evaluation
QCA was performed by means of the CAAS II analysis system (Pie Medical B.V., Maastricht, The Netherlands). In each patient, the stented segment and the peri-stent segments (defined by a length of 5 mm proximal and distal to the stent edge) were analyzed. The following QCA parameters were computed: minimal luminal diameter (MLD), reference diameter, and% diameter stenosis. ABR was defined in every segment as diameter stenosis >50% at follow-up. Late loss was defined as the difference between MLD at post-procedure and MLD at follow-up.
Intravascular Ultrasound Analysis
Post-procedure and follow-up stented vessel segments were examined with mechanical or phased-array IVUS using automated pullback at 0.5 mm per second. A coronary segment beginning 5 mm distal to and extending 5 mm proximal to the stented segment was also examined. A computer-based contour detection program (Curad B.V., Wijk bij Duurstede, The Netherlands) was used for automated 3-D reconstruction of the stented and the peri-stent segments. The lumen, stent boundaries and external elastic membrane were detected using a minimum cost algorithm. The stent volume (SV) and lumen volume (LV) were calculated according to Simpson’s rule. The in-stent neointimal volume was calculated as “SV-LV”. The % obstruction of the stent volume was calculated as in-stent neointimal volume/stent volume _100. Feasibility, reproducibility and inter- and intra-observer variability of this system have been validated in vitro and in vivo13.
Statistical analysis
The primary endpoint and all trial endpoints were analyzed on the per-treatment evaluable population. Acute success was analyzed on the safety population. The per-treatment evaluable population consisted of patients who had no bailout and no major protocol deviations. The data for each patient were reviewed in a blinded manner to determine whether the patient should be included in this analysis population. Analyses based on the per-treatment evaluable population were as “treated”. Patients were included in the treatment arm corresponding to the study stent actually received.
The overall sample size calculation for this trial was determined based on the primary endpoint of in-stent late loss at 6 months and on the following assumptions: a single comparison of active to control; one-tailed t-test, unequal and unknown variances in the 2 groups being compared; α = 0.05; true mean difference between the control group and the treatment group is 0.48 mm. This assumption was made based on the results of VISION Registry (mean late loss = 0.83 mm)14, SIRIUS trial (mean late loss = 0.17 mm)2 and TAXUS IV trial (mean late loss = 0.39 mm)15. Assuming the true mean late loss for the treatment group was 0.35 mm, the difference between the control group and treatment group is calculated as: 0.83 mm – 0.35 mm= 0.48 mm. The standard deviation was assumed to be 0.56 mm in the control group and 0.38 mm in the treatment group (based on the results of VISION Registry study and SIRIUS trial with standard deviation for DES adjusted downward from 0.44 mm to 0.38 mm to take into account of 6-month angiography as opposed to 8-month angiography); approximately 20% rate of lost to follow-up or dropout; approximately 10% of patients with bailout stents; given the above assumptions, enrolling 30 patients per arm (analysis of 22 evaluable patients per arm) would have provided 95% power for comparison. Although the trial was not powered based on the major secondary endpoint, percent volume obstruction at 180 days, enrolling 30 patients per arm (analysis of 22 patients per arm) provides more than 96% power.
Binary variables were compared using Fisher’s exact test. For continuous variables, means and standard deviations were calculated and groups compared using the Wilcoxon’s rank sum test. Time-to-event variables were compared with Kaplan-Meier analysis and the log rank statistic.
Results
A total of 60 study patients were randomized and consecutively enrolled at 9 investigational sites between December 2003 and April 2004. The safety population is composed of these 60 patients. Of the 60 patients, 3 were excluded from the per-treatment population (1 from the everolimus arm and 2 from the bare stent arm) because of bailout stenting (2) and major protocol deviation (1 patient on a heart transplant waiting list from bare stent arm). Hence the per-treatment population includes 56 patients (27 everolimus arm and 29 control) as illustrated in the trial profile (Figure 1).
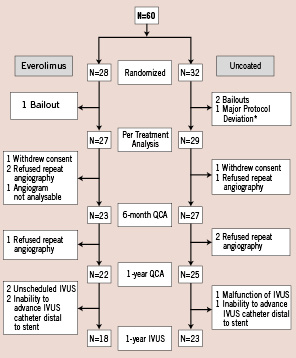
Figure 1. Flowchart of patients. QCA, quantitative coronary angiography; IVUS, intravascular ultrasound.
The control arm and the everolimus arm shared similar demographic characteristics except for patients with hypertension which was significantly higher in the everolimus group than in control (Table 1).
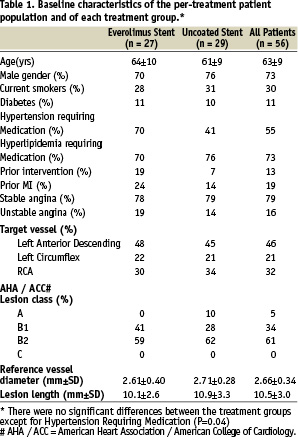
Procedural characteristics were explained previously5.
One-year quantitative coronary angiographic analysis (Table 2)
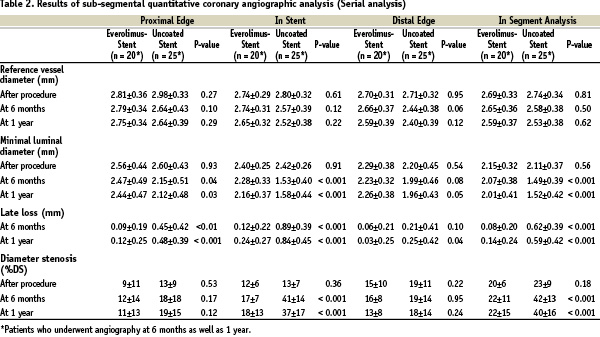
Nine patients did not have qualifying follow-up angiogram up to 1 year for the following reasons: a) patients withdrew from the clinical trial after the 30-day follow-up visit (1 patient in the everolimus arm and 1 in the control arm); b) patients refused (3 in the everolimus arm and 3 in the control arm); c) angiogram was not analyzable (1 in the everolimus arm). Serial angiographic follow-up data, which is reported in this paper, were available in 80.4% (45/56) of the per-treatment population, with 74.1% (20/27) in the everolimus arm and 86.2% (25/29) in the control arm (Table 2). The follow-up in-stent MLD was significantly larger in the everolimus arm than in the control arm and the preservation of MLD between 6 months and 1 year was observed (2.28±0.33 mm at 6 months; 2.16±0.37 mm at 1 year) The mean in-stent late loss and% diameter stenosis were 0.24 mm and 18%, respectively, in the everolimus-stent group, as compared with 0.84 mm and 37%, respectively, in the control arm (p < 0.001 for each comparison). Figure 2 shows the cumulative frequency of in-stent late loss immediately after the index procedure at 6 months and 1 year in each treatment group.
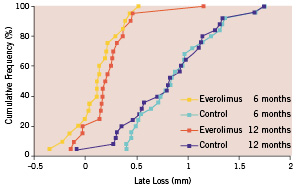
Figure 2. Cumulative frequency of late loss (in-stent) immediately after stenting.
The late luminal loss at both the proximal and the distal edges of the stent was less in the everolimus-stent group than in the control arm (p < 0.001 for proximal and p = 0.04 for distal). The in-segment late loss was significantly less in the everolimus arm than in the bare stent arm (p < 0.001).
One-year intravascular ultrasound evaluation (Table 3)
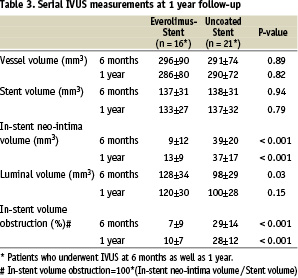
In this 1-year report, data in patients who underwent IVUS at 6 months as well as 1 year were presented to identify the volumetric change in serial IVUS examination. Forty-one patients (18 in the everolimus arm; 23 in the control arm) out of 47 patients with 1-year angiography underwent a 1-year IVUS examination. In the remaining 6 patients, IVUS was not available: 2 were not properly scheduled for IVUS, 2 inability to advance IVUS catheter distal to stent in the everolimus arm; 1 malfunction of IVUS, 1 inability to advance the IVUS catheter distal to the stent in the control arm. Of the 41 patients, 37 patients (16 in the everolimus arm; 21 in the control arm) had serial IVUS data. Everolimus-eluting stent was associated with a significantly reduced degree of in-stent neointimal hyperplasia as well as in-stent% volume obstruction compared to the bare metal stent (13±9 mm3 vs. 37±17 mm3, p < 0.001; 10±7% vs. 28±12%, p < 0.001), reaching a 64% reduction of the in-stent volume obstruction (Table 3). There was no late acquired or persisting stent malapposion observed either at 6 months or at 1 year.
Major adverse events and clinical outcomes
Table 4 provides results of MACE and target vessel failure for the time points of 1 year.
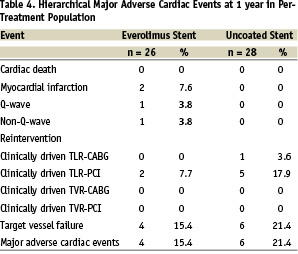
Since the six months follow-up the 1-year results for the everolimus arm included 1 non-Q wave MI due to a spasm during the follow-up IVUS procedure and 2 additional TLRs by PCI. One of these patients had a delayed bailout (TLR) using a non-study drug eluting stent 21 days after the baseline procedure due to a dissection. In the control arm, 1 additional TLR by PCI was observed, this being the patient’s 3rd TLR since the index procedure. The hierarchical MACE rate at 1 year was 15.4% for the everolimus arm and 21.4% for the bare stent arm (p=0.59). The MACE rate for the everolimus group increased from 7.7% (2/26) at 6 months to 15.4% (4/26) at 1 year. Three of the 4 overall MACE events in the everolimus group were non-study-device related events. One Q-wave MI was in a non-target vessel, one TLR was due to dissection during the procedure, and one non-Q-wave MI occurred during follow-up IVUS procedure. Total non-hierarchical clinically-driven TLR rates at 1 year were 7.7% in the everolimus arm and 21.4% in the control arm. No adverse effects related to everolimus or the durable polymer were noted. Kaplan-Meier survival estimates were performed for overall MACE (Figure 3).
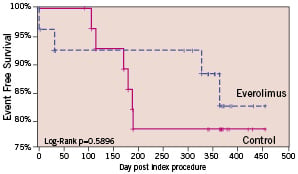
Figure 3. Kaplan-Meier survival curve: MACE. Since the 6-month time point, 1 non-Q wave MI due to a dissection during the follow-up IVUS procedure and 1 clinically-driven additional target lesion revascularization by PCI were observed in everolimus arm. In the control arm, 1 clinically-driven additional target lesion revascularization by PCI was performed.
There was no stent thrombosis observed in both arms out to the 1-year time period.
Discussion
One-year clinical and angiographic follow-up from this trial demonstrates that the polymer-controlled release of everolimus from a coronary stent is safe and effective, with no late adverse effects. The superiority in efficacy, as measured by in-stent late loss, of everolimus-eluting stent as compared to bare stent was sustained at 1 year (71% reduction in late loss). The everolimus arm also maintained its superiority to the bare metal arm in the major secondary IVUS endpoint,% volume obstruction, at 1 year (64% reduction). In addition, the everolimus arm also continued to show significantly lower neointimal volume than the bare stent arm at 1 year (65% reduction).
The current strategy of local drug delivery using sirolimus and paclitaxel is the most promising approach to prevent restenosis, but, at the same time, the strategy has the potential liability for impairing endothelial recovery. Developing new compounds may improve on the potential side effects of the current drug-eluting stents, such as delayed healing with re-endothelialization16 and fibrin17, early18 and late stent thrombosis19. In this trial, neither stent thrombosis nor other adverse effects related to the drug/durable polymer was observed out to the 1-year time point. On the other hand, an invitro study has shown that sirolimus enhances tissue factor in human endothelial cell20. Effect of everolimus on endothelial cell and its similarity or difference compared to sirolimus will have to be investigated.
The significant differences between sirolimus- and paclitaxel-eluting stents have recently been reported to likely exist with regard to angiographic as well as clinical outcomes21,22. “New comers” following these 2 pioneers could be competitors if they can, at least, demonstrate performance as effective as these 2 drug-eluting stents. Studies have suggested that angiographic assessment of late loss is associated with an increased restenosis rate23,24 as well as a higher risk of TLR25. However, it still remains to be determined how to interpret the significance of the slight increase in late loss from 6 months (0.12 mm) to 1 year (0.24 mm) observed in this study stent. Moreover, delayed neointimal growth beyond the first 6 to 9 months has been reported in serial IVUS analyses in some trials as documented in everolimus-eluting stent (in-stent volume obstruction, 7% at 6 months to 10% at1 year), which may raise a concern about potential late catch-up phenomenon of DES26. Recent head-to-head comparative studies between sirolimus- and paclitaxel-eluting stent are still limited to short-term results21,22,25,27-30. Beneficial short-term outcomes do not necessarily translate in long-term efficacy. For example, late catch-up phenomenon has been experienced in vascular brachytherapy31. In this respect, the follow-up period of 1 year still seems relatively short to assess the durable safety and efficacy of one drug-eluting stent. However, neither sirolimus- nor paclitaxel-eluting stent have been associated with gradually increasing MACE over the years32,33. Therefore, we could expect a similar lasting treatment effect of this new eluting stent.
Study limitation
This study with a small patient population provided only safety and efficacy data. Two larger single-blind, randomized controlled studies (The SPIRIT II and SPIRIT III) further evaluating this study stent compared to the paclitaxel-eluting stent for the treatment of coronary artery disease are under way.
Conclusions
At 1 year, this trial demonstrated that the treatment effect observed at 6months was sustained at 1 year for everolimus-eluting stent. The in-stent and in-segment late loss in the everolimus arm was reduced by 71% and 78% compared to those in the bare metal arm, respectively. These observations were consistent with IVUS measurements. The 1-year results showed a reduction of neointimal volume by 65% as compared to bare metal stent. A small increase in % volume obstruction in event-free patients was observed from 6 to 12 months, but is considered clinically insignificant. Both the angiographic and IVUS measurements showed that the patency of the target vessel treated with everolimus-eluting stent was maintained at 1 year.
Acknowledgement
The authors are grateful to the invaluable assistance of Stan Fink and Danny Detiège (Guidant, Santa Clara and Guidant Europe) for their valued assistance in reporting this study.

