Abstract
Aims: The one year clinical benefit of various doses and release durations of paclitaxel eluted from an erodable polymer has not been evaluated so far.
Methods and results: Conor paclitaxel-eluting stents have intra-stent wells in which drug and polymer are deposited. Stents with six different release formulations (dose: 10 µg or 30 µg, duration: 5, 10 or 30 days, direction: mural or bidirectional) were implanted in 6 patient cohorts. Clinical follow-up was conducted at 4 and 12 months. Quantitative angiography and IVUS were performed at 4 months, and additional angiographic and IVUS follow-up were performed for groups D5 (10µg/30days/mural) and D6 (30µg/30days/mural), as they had shown the most favorable results at 4 months. At one year, the lowest major adverse cardiac event rates were observed in the slow release (30 day) group (5.1% in D5 and 6.9% in D6). One-year in-stent late loss was 0.52±0.34 mm in D5 and 0.36±0.50mm in D6 (p=0.20) while neointimal area was 0.99±0.54 mm2 in D5 and 0.77±0.92 mm2 in D6 (p=0.42). Corresponding in-stent binary restenosis at one year was 0% and 5.6% respectively (p=0.36).
Conclusions: Patients who received the slow release formulation stent had better clinical outcome at one year than those who received the fast release formulation. However, the effect on neointimal suppression requires investigation in a larger population to determine whether the high dose formulation confers an additional clinical benefit.
Introduction
Drug-eluting stents consist of a drug, a polymer, and a stent platform. Several drugs with durable or erodable polymers have been tested in clinical trials and show that drug-eluting stents significantly inhibit neointimal growth compared to bare metal stents1-4. However, the most effective drug dose and pharmacokinetic release formulation have not been evaluated thoroughly in humans.
The Paclitaxel In-Stent Controlled Elution Study (PISCES) has demonstrated that kinetic variations play a key role in the efficacy of a drug-eluting system5. At 4 months, the inhibition of in-stent neointimal hyperplasia was better in the slow release groups compared to the fast release groups. The present study evaluates (1) the one-year clinical outcome in all 6 groups and (2) neointimal growth in the two slow release groups, using serial quantitative coronary angiography (QCA) and intravascular ultrasound (IVUS) analysis; in order to understand the long-term impact of drug dose and pharmacokinetic release.
Methods
Patient selection
The PISCES trial was a prospective, multi-center, sequentially enrolled, non-randomized, open-label trial in which patients were treated with a Conor paclitaxel-eluting stent in one of six different release formulations, and the results of each group was compared (Table 1). The study device and protocol have been described previously5,6. In brief, 191 patients with single de novo lesions with a reference diameter of 2.5-3.5 mm and a lesion length that could be covered by a single 17mm stent were enrolled. Conor drug-eluting stents were loaded with 10 or 30 µg of paclitaxel within a bioresorbable polylactide-co-glycolide (PLGA) matrix. The drug and polymer were deposited in the wells. The in-vitro drug release period was either 10 or 30 days. The PLGA polymer is fully erodable and neither polymer nor drug is retained in the stent after several months of implantation.
Follow-up and endpoints
The study protocol required all patients to have follow-up clinic visits with an electrocardiogram (ECG) at one, four and twelve months. An independent clinical event committee adjudicated clinical events and ECGs. Quantitative angiography and IVUS were performed at 4 months. Clopidogrel was discontinued per protocol at 6 months following stent implantation.
Additional angiographic and IVUS follow-up was performed at 12 months in groups D5 and D6 which showed the best results at 4 months (Figure 1)5,6.
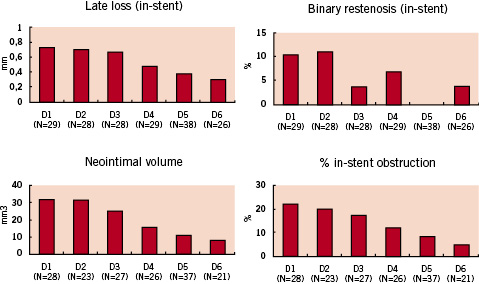
Figure 1: 4-month QCA and IVUS results.
The safety endpoint of the present study is a composite of major adverse cardiac events (MACE) defined as cardiac death, Q-wave or non-Q-wave myocardial infarction, and target lesion revascularization (TLR) at 12 months. If the cause of death was undetermined, it was categorized as cardiac death. Myocardial infarction (MI) was diagnosed by a rise in the creatine kinase level to more than twice the upper normal limit with an increased creatine kinase-MB accompanied by new abnormal Q-waves in the surface electrocardiogram (Q-wave MI) or not (non-Q-wave MI). TLR was defined as revascularization of the stented and the peri-stent segments (5mm proximal and distal). Target vessel revascularization (TVR) was defined as revascularization due to narrowing (>50% diameter stenosis) of any portion of the target vessel outside the peri-stent segment but was not included as an event in the MACE rate.
The efficacy endpoints included the in-stent and peri-stent (in-stent + 5 mm proximal edge + 5 mm distal edge) angiographic late loss and binary restenosis rate as well as percent in-stent volume obstruction as determined by quantitative intravascular ultrasound (IVUS).
Quantitative Coronary Angiography (QCA) evaluation
The quantitative ultrasound and coronary angiographic (QCA) analyses were performed by an independent core laboratory that remained blinded to treatment allocation (Cardialysis, Rotterdam, The Netherlands). Quantitative coronary angiography was performed by means of the CAAS II analysis system (Pie Medical BV, Maastricht, The Netherlands). In each patient, the in-stent and peri-stent segments were analyzed. Binary restenosis was defined in every segment as diameter stenosis >50% at follow-up. Late loss was defined as the difference between MLD post-procedure and MLD at follow-up
Quantitative Intravascular Ultrasound (IVUS)
Post-procedure and follow-up stented vessel segments were examined with intravascular ultrasound (Cardio Vascular Imaging System, CVIS, Sunnyvale CA, U.S.A.) using an automated pullback at 0.5 mm per second. A computer-based contour detection program was applied using CUARD QCU analysis software (Cuard BV, Wijk Bij Duurstede, The Netherlands) for 3-D reconstruction of the stented and adjacent segments7,8. The intrastent neointimal area was calculated as the stent area minus lumen area, and plaque area outside the stent was calculated as the vessel area minus stent area. The percentage in-stent volume obstruction was calculated as intrastent neointimal volume/stent volume*100.
Statistical analysis
The analyses of MACE, angiographic and IVUS parameters were per protocol based, in patients who received the allocated Conor paclitaxel-eluting stents. Continuous variables are expressed as mean±standard deviation. Discrete variables are presented as percentages. For patient demographics, the following tests were applied to calculate the differences among the six groups: F-test from an analysis of variance, two-sample t-test, likelihood ratio chi-square test, Fisher’s exact test and Cochran-Mantel-Haenszel test. For QCA and IVUS parameters, continuous variables were compared between groups D5 and D6 with the Student t test, and comparisons between 4 months and 12 months within the same group were performed with a paired t test. The Fisher exact test was used for categorical variables. All statistical tests were two-tailed, and p values less than 0.05 were considered statistically significant.
Results
Patient and lesion characteristics
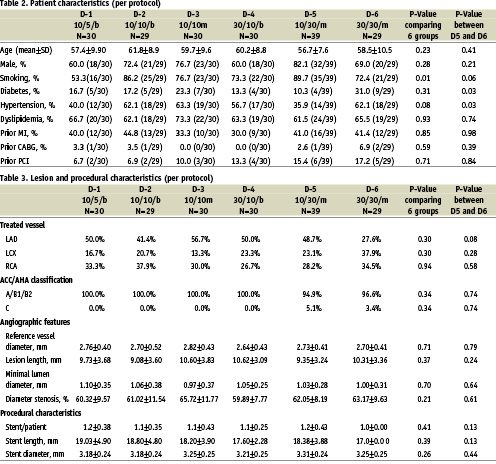
In the PISCES trial, 191 patients were enrolled. The investigational device could not be implanted in four patients. In total, 187 patients were treated with one of the six different formulations of paclitaxel-eluting Conor stents. The average age was 59.1±9.2 years and the prevalence of diabetes was 18.2% in the total population. The baseline demographic and angiographic data was similar among the six groups, except for the incidence of a positive smoking history (Tables 2 and 3).
Clinical events
Clinical follow-up was complete for all patients at one year (Table 4). At four months, the slow release groups had a relatively lower incidence of MACE compared to the fast release groups (2.6% in D5 and 3.4% in D6). This tendency did not change at one year (5.1% in D5 and 6.9% in D6; Figure 2). Between 4 months and 1 year, a MACE occurred in two patients in the slow release groups (D5 and D6): one patient in D5 suffered a non Q-MI due to a non-TVR (maximum CK level of 356 U/L), and one patient in D6 had diffuse in-stent restenosis (binary restenosis of 68%) at 4-month angiographic follow-up with a positive exercise tolerance test. This patient was placed on the waiting list for a repeat intervention. Two weeks after the angiography, she was admitted with a Q-wave MI (maximum CK level of 1687 U/L) and underwent re-catheterization which demonstrated total occlusion at the inlet of the stent. This patient was subsequently treated with a sirolimus-eluting stent. Notwithstanding this patient who had angiographic restenosis, a positive functional test for ischemia but delayed re-intervention at the 4 month follow-up, there were no instances of abrupt, delayed stent thrombosis in the PISCES patients.
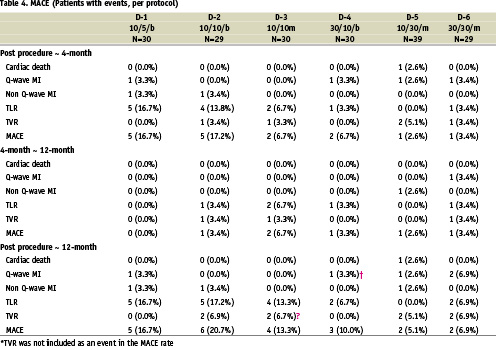
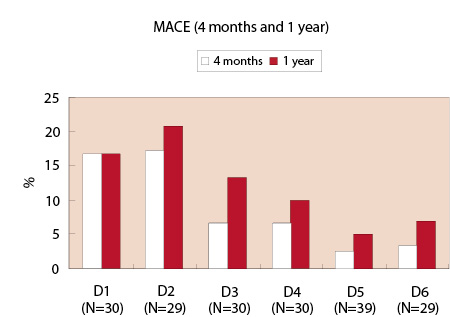
Figure 2: 4-month and 1-year MACE.
Serial QCA analysis
A total of 50 patients (74%) in groups D5 and D6 underwent serial QCA analysis at 4 months and 1 year. The baseline and post-procedure QCA data were similar in the two groups (Table 4). At 4 months, in-stent late loss was not significantly different between D5 and D6, although in-stent late loss was lower in D6 than in D5 (0.32±0.40 mm versus 0.40±0.32 mm respectively, p=0.43). From 4 months to 1 year, the late loss increased in both groups but the trend remained in favor of D6; no statistical difference between the two groups could be established although D6 showed a lower in-stent late loss at 1 year (0.52±0.34 mm in D5 and 0.36±0.50 mm in D6, p=0.20). Overall peri-stent binary restenosis at 1 year was observed in one patient in each group (3.1% in D5 and 5.6% in D6, p=1.00).
Serial IVUS analysis
A total of 45 patients (66%) underwent serial IVUS analysis at 4 months and 1 year. The IVUS results showed no statistical differences between groups D5 and D6 (Table 5). The percent in-stent obstruction at 1 year was 12.46±7.60 in D5 and 8.37±9.10 in D6 (p=0.12). However, in D5, the neointimal area increased significantly from 4 months to 1 year (delta=0.38mm2, p=0.0003) whereas the difference between 4 months and 1 year in D6 failed to be significant (delta=0.21 mm2, p=0.36, Figure 3).
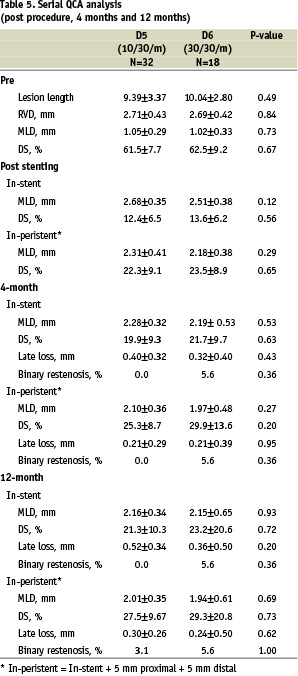
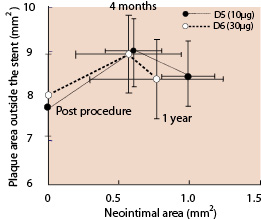
Figure 3. Correlation between neointimal area and peri-stent area over 2 years. Error bars indicate 95% CI. P values for Neointimal area D5: Between post procedure and 4 months: <0.0001 Between 4 months and 1 year: 0.0003 D6: Between post procedure and 4 months: 0.029 Between 4 months and 1 year: 0.36 P values for plaque area outside the stent D5: Between post procedure and 4 months: <0.0001 Between 4 months and 1 year: 0.045 D6: Between post procedure and 4 months: 0.0015 Between 4 months and 1 year: 0.0023
At 4 months, significant expansive remodeling (an increase in the plaque area outside the stent) was observed in both groups (7.75±1.93 mm2 vs 9.09±2.45 mm2, p<0.0001 in D5; 8.04±1.76 mm2 vs 8.95±1.67 mm2, p=0.0015 in D6). Between 4 months and 1 year, a significant regression of the expansive plaque area outside the stent was observed in both groups (9.09±2.45 mm2 vs 8.46±2.15 mm2, p=0.045 in D5; 8.95±1.67 mm2 vs 8.37±1.74 mm2, p=0.0023 in D6).
Discussion
The main findings of this study are the following: first, the slow release (30 day) groups have better clinical outcomes at 1 year compared to the fast release (5 or 10 day) groups. Second, compared to the low dose (10 µg) group, the high dose (30 µg) group had lower late loss and neointimal volume at one year without statistically significant difference. Third, between 4 months and 1 year, modest neointimal growth continued in both the low and high dose groups without new instances of in-stent angiographic restenosis or target lesion revascularization, and this neointimal growth was statistically significant in the low dose group only. Fourth, plaque outside the stent increased during the first 4 months following Conor paclitaxel-eluting stent implantation, but by 1 year it had partially regressed in both the low and high dose groups.
Various drug elution processes have reported conflicting clinical results3,9-12. In the SCORE trial, QuaDDS stents with a total of 4000 µg paclitaxel and a durable acrylate polymer were found to have an unacceptable safety profile9. In the DELIVER trial, the Multi-Link PENTA stent with a coating of 45-150µg paclitaxel (a dose density of 3.0µg/mm2 stent surface area) without a polymer showed no impact in reducing clinical revascularization or restenosis compared to bare metal stents, presumably due to the rapid elution of the drug10. In the ELUTES and ASPECT trials, the high-dose non polymer paclitaxel-eluting stent has the better outcomes compared to the low dose group11,12. In the TAXUS IV trial, a slow-release (7.5% at 30 days), polymer-based paclitaxel eluting stent with a total of 106 µg paclitaxel (a dose density of 1.0 µg/mm2 stent surface area) eluted from a durable polymer showed significantly better inhibition of neointimal growth and clinical outcomes when compared to bare metal stents3. In the light of this study and other paclitaxel–eluting stent trials, the drug release profile is a key factor in the clinical efficacy, and drug-eluting stents with a slow release formulation seem to be more efficient. In the present study, the slow release groups demonstrate the best 1-year clinical outcomes, mainly due to better outcomes in the first 4 months. The drug and polymer are completely removed from the wells after several months, thus this novel drug-eluting stent may potentially preclude the chronic vessel reaction usually observed with a durable polymer and persistent drug on the stent13,14. Further, there were no instances of late stent thrombosis. Though more data from larger studies is required to draw definitive conclusions, these results support the hypothesis that complete drug elution and polymer resorbtion may confer safety benefits with respect to delayed thrombosis.
In this study, the expansive vessel remodeling observed at 4 months seems similar to the remodeling observed after implantation of Taxus polymeric paclitaxel-eluting stents15. The expansive vessel shrinkage observed at 1 year also suggests that the chronic vessel reaction to mechanical injury and biological reaction to the drug and polymer have subsided in that period of time.
However, the chronic vessel reaction inside the stent differs from the reaction observed behind the stent struts. Although this study showed a regression of tissue growth outside the stent between 4 months and 1 year, compaction of neointima was not observed in either the low dose or the high dose group over the same time period. The precise reason for this phenomenon is unclear. One might hypothesize that this could be a result of different tissue composition inside and outside the stent. The tissue growth inside the stent is composed of smooth muscle cells in a proteoglycan rich matrix, whereas the tissue growth behind the stent struts consists of several components: 1) intracellular matrix and cell proliferation such as smooth muscle cells and lymphocyte cells, 2) oedema due to mechanical injury and biological reaction against the drug, polymer and stent, and 3) growth or regression of existing atherosclerotic plaque. Thus, the direction of volumetric change (regression or expansion) from 4 months to 1 year may not be similar inside and outside the stent.
In this study, the actual late loss and neointimal area were smaller in the 30 µg group than in the 10 µg group. These differences were not statistically different and did not influence the clinical outcomes. The 10 µg paclitaxel dose may be sufficient to suppress neointimal growth in humans at least for a period of one year. It may also be argued that the sample size is too small to detect a biological difference between the low and the high dose.
In animal studies, paclitaxel polymer coated stents have been found to inhibit in-stent neointimal growth but with signs of delayed intimal healing at 28 days, such as fibrin deposition, inflammation and increased cellular proliferation. By 90 days, local toxicity associated with paclitaxel resolves but in-stent neointimal growth suppression is no longer present16. In humans following bare metal stent implantation, the neointima does not keep growing beyond 6 months and instead begins to regress due to the replacement of water-trapping proteoglycans (hyaluronan and versican) by decorin and type I collagen17. In this study, compaction of neointima was not observed and it is unknown whether neointimal tissue will keep growing or stop beyond one year. Further follow-up is warranted to evaluate the long-term efficacy of these devices and to find the best elution period and drug dose.
Limitations
At one year, 26% and 34% of the patients in the groups of D5 and D6 respectively did not undergo serial invasive QCA or IVUS follow-up evaluation. Following completion of patient enrolment, the protocol was subsequently amended to allow one year angiographic and IVUS follow-up, necessitating a new informed consent. Patients who did not undergo one-year angiography reported no anginal symptoms at one year. The sample sizes for groups D5 and D6 were insufficient to detect a difference in outcome between the low and high doses in the slow release formulation. However, they served as the basis for the development of a large randomized trial (the EuroSTAR trial) which is evaluating both doses (10 µg and 30 µg per 17mm stent) of slow-release paclitaxel using the reservoir-based technology on an ultra-thin cobalt-chromium stent in 270 patients.
Conclusions
The PISCES trial suggests that the pharmacokinetics of drug-eluting stents is important for both neointimal suppression and for clinical outcomes at 1 year. The slow release (30 day) formulation had better clinical outcomes compared to the fast release (5 or 10 day) formulation. The drug dose (10 µg or 30 µg) did not seem to influence the amount of neointimal suppression but the sample sizes in this pilot dose-finding study were insufficient to detect a beneficial difference in dose.
Acknowledgement
The authors thank Patty Hevey and Louise Gambone for data management and careful review of the manuscript.

