Abstract
Aims: In this first multicentre study we assessed the safety and efficacy of percutaneous transendocardial skeletal myoblast injection as a stand alone procedure in congestive heart failure patients.
Methods and results: 15 patients (14 male), age 63±7 (Mean±SD), NYHA class 2-4 were injected with 216±119 cells (81±19% Desmin+) using a NOGA or fluoroscopy guided injection catheter. The cells were injected in the scarred regions 6±4 years after myocardial infarction as a stand alone procedure. After treating the first 6 patients, the protocol was amended to require that remaining patients be fitted with an ICD prior to the cellular cardiomyoplasty procedure. Holter monitoring, ECG and ICD readings were obtained at multiple intervals. Stress echocardiography and LV angiography was performed at baseline, 3, 6 and 12 months post procedure.
After 1 year follow-up 13 patients were still alive. Patient # 6 died suddenly 9 days post procedure. Patient #15 (ICD patient) survived an electrical storm 12 days post procedure, but died 2 days later due to cardiogenic shock. Two non-ICD patients received an ICD because of observed ventricular arrhythmias. It remains unknown whether these events are directly related to the cell injections.
LV ejection fraction (%) changed from 34.4±10.3 to 36.6±10.4 (baseline versus 12 months FU, p=0.26). Wall motion score index improved both at rest (3.0±0.5 to 2.7±0.7, p=0.049) and under low-dose dobutamine stress (2.8±0.4 to 2.5±0.6, p=0.07, baseline versus 12 months FU).
Conclusion: Percutaneous autologous skeletal myoblast injection is feasible, resulting in wall motion and functional class improvement, but is potentially associated with an increased risk for ventricular arrhythmias. Randomised studies are needed, however, to further assess overall safety, efficacy and the potential for initial increased risk for arrhythmia following cell injection in these high-risk patients.
Introduction
Myocardial infarction is associated with irreversible loss of cardiomyocytes and cardiac function. Despite early reperfusion and optimal pharmacological treatment the late sequels of myocardial infarction often lead to heart failure1. Cell transplantation for myocardial repair has emerged as a promising therapy for patients after myocardial infarction. Cell based myocardial repair is currently focussed on the subacute phase of myocardial infarction or the chronic heart failure stage after myocardial infarction.
In the subacute phase, cell transplantation is mainly investigated via intracoronary infusion of bone marrow derived cells into the infarct-related artery2-5. Whereas in the chronic heart failure patients, cell transplantation is mainly carried out via direct intramyocardial injection of autologous skeletal myoblasts in the scar and its surrounding tissue.
Recent studies have shown that following the surgical intramyocardial injection, autologous skeletal myoblasts survive, engraft, demonstrate myotube formation and express the slow isoform of the myosin heavy chain6,7. Although many pre-clinical studies8-15 and preliminary clinical studies16-19 show feasibility, safety and efficacy have not yet been established.
We report on the first stand-alone, multicentre study utilizing the percutaneous approach for the delivery of autologous skeletal myoblasts in post myocardial infarction chronic heart failure patients.
Methods
Objectives
The primary objective of this pilot study was to assess the feasibility and safety of the transendocardial delivery of autologous myoblasts at 6 and 12 months. The secondary objective was to assess improvement of left ventricle function by iterative investigations of several functional parameters at baseline, 1, 3, 6 and 12 months. The protocol was approved by the local Medical Ethics Committee and written informed consent was obtained from all patients. Safety aspects of the trial were monitored by an independent DSMB.
Patient selection
Only symptomatic patients NYHA functional class > 2 under optimal medical therapy were selected. All patients had a large myocardial infarcted area and a depressed left ventricle function (left ventricle ejection fraction (LVEF) between 20–45% as measured by radionuclide radiography or ventricular angiography). Myocardial infarction had to be older than 4 weeks at the time of cellular implantation. The presence and location of a myocardial scar was defined by akinesia or dyskinesia at rest by echocardiography and LV angiography and/or by magnetic resonance imaging (MRI). Absence of ischemia was assessed by dobutamine stress echocardiography. Target region wall thickness >5 mm in thickness as measured by echo or MRI scan was required. Patients with positive serology test results on HIV, Hepatitis B, C or syphilis were excluded from the study.
Initially patients with a history of syncope or potential candidates for Implantable Cardioverter Defibrillator (ICD) therapy were not considered eligible for the study. Two patients among the first six enrolled presented with serious arrhythmic adverse events, which necessitated interruption of the study. Following consultation with the DSMB, it was recommended that an amendment to the study be made to enrol only patients fitted with an ICD. This recommendation was implemented in the remaining 9 patients to be enrolled.
Muscle biopsy
Biopsy of the quadriceps muscle was done under local anaesthesia. On average 11.5 grams (range 3.0-19.8 grams) of muscle biopsy was excised through a 10 cm long surgical incision. All 15 biopsy procedures were uneventful and done on an out-patient basis. Biopsies were placed in a bottle containing a proprietary solution designed to preserve the biopsy during controlled shipment. The bottle was put in an insulated thermobox with frozen and refrigerated gelpacks to maintain temperatures between 2°C to 8°C during transit. The transport conditions were monitored by the use of a programmable temperature monitor (Sensitech, Beverly MA, USA). The container was sent to a cGMP cell culturing facility (BioWhittaker, Cambrex Bio Science Corp, Walkersville MD, USA) for myoblast cell isolation and expansion. Average transport duration was 41 hours (range 35-50 hours), with no temperature excursions noted.
Cell culturing process
Upon receipt at the culturing facility, biopsies were processed according to Bioheart, Inc MyoCell™ protocols. The biopsy was finely minced and then dissociated using digestive enzymes. The dissociated tissue was washed several times and filtered until a single cell suspension was achieved. Expansion of the culture was initiated when the cells were plated into sterile tissue culture flasks containing growth media specifically for skeletal muscle myoblasts. The media was changed at regular intervals and cells were harvested and replated according to cell confluence parameters. Final harvest occurred after 3-5 passages. Myoblasts were identified using an immunohistochemical marker specific for desmin (DAKO) to identify cells committed to myogenic differentiation. Cell lot release specifications (cell viability, cell identity and sterility tests) were established prior to start of the study; if these specifications were not achieved, the patient would be approached for re-biopsy and re-initiation of the culture process. Three patients required a second biopsy due to desmin staining results falling below the lot release criteria. In these patients, a pre-stimulation procedure was performed using multiple needle punctures of the muscle 3 days prior to the biopsy procedure in order to increase the percentage of myoblast cells in the muscle biopsy7.
After a culturing period of 17 days (range 14-19 days), the harvested cells were mixed with a specially formulated transport media, transferred into a sterile 30 ml bag and sent to the clinical investigation site. Cells were subject to the same controlled shipping conditions as biopsies. Transit time averaged 62 hours (range 24-96 hours) with no temperature excursions noted. The cells had been previously validated for a 96 hour shelf life under controlled conditions.
Transplantation procedure
The cell transplantation procedure was scheduled the day of or day after arrival of the cells and was performed in the cardiac catheterization laboratory. Access was obtained via the femoral artery and 100 IU/kg heparin was given. Target ACT was between 250-300 sec. and regularly checked every half hour. After a coronary and biplane LV angiogram (LAO 60° and RAO 30°) was made, an outline of the LV chamber was drawn on transparent mylar sheets which were taped to the fluoroscopy monitors. Transendocardial injections were made using the 8F MyoCath™ fluoroscopy-guided needle injection catheter (Bioheart, Weston, Florida, USA) or the NOGA™-guided needle injection catheter (Biosense-Webster, Waterloo, Belgium). The fluoroscopy-guided needle injection catheter was placed in the left ventricle (retrograde) across the aortic valve. Based on visualization of the left ventricular angiogram and previously obtained stress echocardiography results, the injection catheter was directed towards the scarred target area guided by fluoroscopy in RAO and LAO projection. Depending on the average wall thickness of the target region, the needle length was set to 4.5-6.0 mm when the catheter tip was deflected to a 90° curve. We refrained from transendocardial injections into areas with a known wall thickness of less than 5 mm by echocardiography, per protocol. After visual and tactile confirmation of endocardial contact was made, the needle was extended. The occurrence of extra ventricular beats after deployment of the needle provided additional proof of intramyocardial penetration of the needle. After re-occurrence of a regular rhythm, the transendocardial injections was made. Injection sites were subsequently marked on the transparent sheets to map the areas of injection within the scar region.
The NOGA™-guided injection procedure has previously been described in detail17. In short, an electro-mechanical NOGA™ map of the left ventricle was obtained using a 7F NOGASTAR™ catheter (F-curve) connected to the NOGA™ console. Areas exhibiting low voltages and linear local shortening (UV < 4 mV and LLS < 4%) on the NOGA™ map were considered as the target areas for treatment, provided these areas were geographically concordant with the scar areas as assessed by the pre-procedural DSE and LV angiogram. With an 8F MyoStar™ injection catheter (Biosense-Webster), transendocardial injections were made. By connecting a 1 ml luer lock syringe to the injection port, the catheter was pre-loaded with the skeletal myoblast solution. After establishing stable endomyocardial contact on the NOGA map and under fluoroscopy, the needle was advanced manually, often causing extra ventricular beats. Once a regular ECG rhythm resumed with continued stable endomyocardial contact, injections were made. Injection sites were marked on the NOGA™ map as well as on the transparent sheets attached to the fluoroscopy screen.
In all cases, 0.3 ml (approximately 15 million cells) was injected at each injection site. Spacing between injection sites was approximately 1.0 cm, with an attempt being made to fully cover the areas of scar. On average 18±7 (mean±SD) injections per patient were made. After all injections were complete, patients were monitored by ECG for 18 hours, during which time cardiac enzyme levels were checked twice at 6-8 hours intervals.
Methods of assessment
Twenty-four hour ambulant ECG monitoring and a dobutamine stress echocardiography (DSE) were performed at baseline, 1, 3, 6 and 12 months follow-up. DSE procedures were performed as previously described17 with a HP Sonos 5500 imaging system equipped with a second harmonic imager to optimize endocardial border detection. After baseline echocardiography, dobutamine was infused at a starting dose of 5 µg/kg/min for 5 min, followed by 10 µg/kg/min for 5 min (low-dose stage). Dobutamine was then increased by 10 µg/kg/min every 3 minutes up to a maximum dose of 40 µg/kg/min. Atropine (1-2 mg) was then added at the end of the last stage if the target heart rate had not been achieved. Images were acquired continuously and recorded on tape at the end of every dose-step. The baseline, low-dose, peak stress, and recovery images (standard apical and short-axis views) were additionally displayed in a cineloop format. Wall motion was scored according to the criteria of the American Society of Echocardiography by 2 independent and experienced reviewers.
LV volume and ejection fractions were assessed by biplane LV angiography at baseline, 3, 6 and 12 months follow-up. LV angiography was performed in (biplane) LAO 60° and RAO 30° projections with a 100 ml sphere calibration filmed in the isocenter. Quantitative analysis of the LV angiogram was independently performed by the angiographic core lab at the Cleveland Clinic, Cleveland, Ohio, USA. Optional Tc99m labelled erythrocytes radionuclide scintigraphy was performed at baseline, 3 and 6 months follow-up.
Holter recordings and ICD data
For the first 6 patients of the study, 7-lead 24 hour holter recordings (Rozinn Electronics Inc.; Glendale, New York, USA) were performed at screening and at 1, 3, 6 and 12 months post injection. In the remaining 9 ICD patients, 7-lead 24 hour holter recordings were done at screening, 1 day prior to injection and at 1, 2, 3 weeks as well as 1, 3, 6 and 12 months post injection. Review of patient ICD data was performed during the screening and biopsy procedure visits as well as pre-injection and at 1, 2, 3 weeks and 1, 3, 6, 12 months post injection. Analysis of the holter and ICD data was performed at the Cardialysis core lab (Rotterdam, The Netherlands). Stored electrograms were visually analyzed using a standardized method. Ventricular tachyarrhythmias were defined by sudden increases in heart rates combined with changes in electrogram morphology compared with baseline rhythm. Fast ventricular tachycardias were defined by rates greater than 150 bpm. In-hospital telemetry recording was performed up to discharge, depending upon the occurrence of arrhythmias.
Statistics
Analysis of variance was performed using linear and/or mixed models for the comparison of wall motion and ventricular angiography parameters at baseline, 3, 6 and 12 months. A two-sided t-test was performed for the comparison of radionuclide ejection fraction between baseline and 3 or 6 months.
Results
Fifteen patients were enrolled in the study (14 male) with an average age of 63±7 years. Patient characteristics are summarized in Table 1.
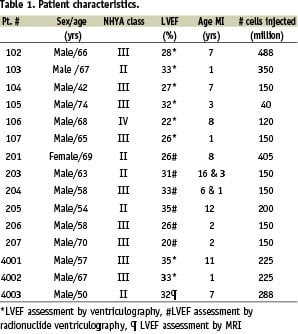
In total, 216±119 million cells were injected with an average Desmin staining of 81±19%. No procedural complications occurred apart from 2 sustained VT episodes during NOGA™ mapping and before cell injection, which were resolved by overpacing and electric cardioversion.
In 2 patients a mild rise (less than 2x UNL) in CK/MB or Trop T was noted post procedure with no associated adverse events. No new Q wave or LBTB on 12 lead ECG was noted during follow-up.
In all 15 cases the in-hospital stay (4±1 days) was uneventful.
Adverse events
Serious adverse events occurred in 4 patients. Two patients died shortly after the cell transplantation procedure (9 and 14 days, respectively). The first patient, who belonged to the first cohort of non ICD patients, died suddenly after complaining of dizziness. Cause of death is unknown and is possibly related to an arrhythmic event. The second patient, who belonged to the ICD cohort, died of cardiogenic shock following an electrical storm. In two other non-ICD patients, symptomatic ventricular arrhythmias were observed 18 days and 14 weeks post cell transplantation. These patients subsequently received an ICD. In one other patient incidental sustained VT runs were observed 28 days post procedure, which were successfully treated by short-term administration of amiodarone.
Holter and ICD monitoring
Holter monitoring
In the first 6 non-ICD patients, no significant ventricular arrhythmias were observed on the holter recordings pre or post injection. In 5 of the 9 ICD patients, pre-injection holter recordings showed a total of 28 episodes of non-sustained VT; 25 episodes were slow (freq. 111- 130 bpm) and 3 episodes were fast (freq. 156 -191 bpm). Fast episodes occurred in 3 patients. Post implant, 8 of the 9 ICD patients showed 184 episodes of ventricular tachyarrhythmias. Two of these patients showed fast tachyarrhythmia’s (180-232 bpm); one of them experienced 130 episodes during an electrical storm.
ICD monitoring
Pre-injection ICD monitoring up to 12 months prior to injection (range 2-12, mean 7 months) showed that 1 patient (#104) had 12 events which triggered ICD therapy. Post injection, ventricular events triggering ICD therapy occurred in 3 patients (#105, #107, #204); one patient at 1 week and in 2 patients at 2 weeks post injection. It is noteworthy that subsequently none of remaining patients needed further ICD therapy over a period of 12 months.
Angiographic data
Serial angiography at baseline, 3, 6 and 12 months post procedure shows a significant decrease in end diastolic volume and trends towards reduction in end-systolic volume with a concomitant increase in Ejection Fraction (EF), though these trends failed to reach statistical significance by variance analysis (Figures 1a-c).
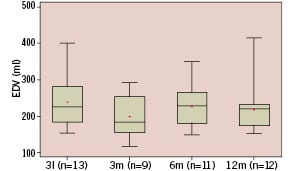
Figure 1a. Whisker box plot representation of end diastolic volume at baseline (n=13, 238±75 ml {mean±std}), 3 months (n=9, 198±59 ml), 6 months (n=11, 227±58 ml) and 12 months (n=12, 219±68 ml). Analysis of variance showed a significant decrease (p=0.009) in end diastolic volume.
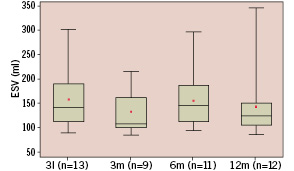
Figure 1b. Whisker box plot representation of end systolic volume at baseline (n=13, 158±66 ml {mean±std}), 3 months (n=9, 133±47 ml), 6 months (n=11, 155±59 ml) and 12 months (n=12, 143±69 ml). Analysis of variance showed a not significant decrease (p=0.56) in end systolic volume.
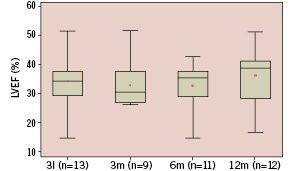
Figure 1c. Whisker box plot representation of left ventricular ejection fraction at baseline (n=13, 34±10% {mean±std}), 3 months (n=9, 33±8%), 6 months (n=11, 33±9%) and 12 months (n=12, 37±10 ml). Analysis of variance showed a trend (p=0.26) towards an increase of LVEF.
Stress echo
A statistically significant improvement in wall motion score at rest was observed at 12 months follow up (3.0±0.5 to 2.7±0.7, p=0.049) with a clear trend towards improvement observed with low- dose dobutamine stress echocardiography (2.8±0.4 to 2.5±0.6, p=0.07, baseline versus 12 months FU) (Figures 2a,b).
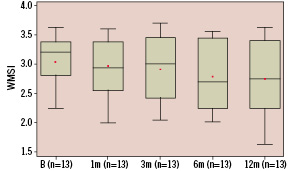
Figure 2a. Whisker box plot representation of wall motion score index in rest at baseline (n=13, 3.0±0.5 {mean±std}), 1 month (n=13, 3.0±0.5), 3 months (n=13, 2.9±0.6), 6 months (n=13, 2.8±0.6) and 12 months (n=13, 2.7±0.7). Analysis of variance showed a significant improvement (p=0.049) of the wall motion score index.
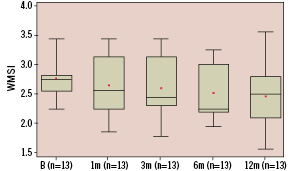
Figure 2b. Whisker box plot representation of wall motion score index during low dose dobutamine stress at baseline (n=13, 2.8±0.4 {mean±std}), 1 month (n=13, 2.7±0.5), 3 months (n=13, 2.6±0.5), 6 months (n=13, 2.5±0.5) and 12 months (n=13, 2.5±0.6). Analysis of variance showed a trend towards improvement (p=0.07) of the wall motion score index.
Radionuclide angiography (MUGA) data
MUGA was only performed in 5 patients. Compared to baseline, at three and six months follow up global ejection fractions remained stable (30±6 to 30±7 and 29±8%, respectively; baseline versus 3 months FU, p=1.0 and baseline versus 6 months FU, p=0.83) (Figure 3).
Functional class data
At baseline, average NYHA functional class was 2.8 and continuously improved to 1.9 NYHA at 12 months follow-up (Table 2).
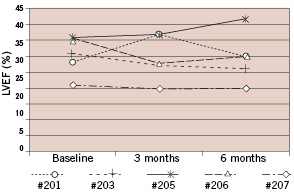
Figure 3. Radionuclide left ventricular ejection fraction results at baseline, 3 months and 6 months of 5 patients treated in 1 center. The LVEF remained unchanged, baseline versus 3 months p=1.0, baseline versus 6 months p=0.83).
Discussion
This multicentre pilot study sought to evaluate the feasibility and safety of the percutaneous endocardial transplantation of autologous skeletal myoblasts in chronic heart failure patients. In parallel with the transendocardial approach in this study, another study (under a separate protocol) was conducted to evaluate the feasibility and safety of the transvenous injection of autologous myoblast cells. During the conduct of these studies, two deaths, one in each study, occurred within in couple days of eachother, triggering the study steering committees and sponsor to consult the independent DSMB. The DSMB advised to temporarily hold enrolment in both studies and requested from a panel of electrophysiologists a detailed analysis of the events associated with each death. Following this analysis the electrophysiology panel and DSMB recommended the following safety measures be undertaken prior to reinitiating enrolment in the studies: inclusion of only patients implanted with ICD, more rigorous ECG and holter monitoring pre and post implantation, 12-lead ECG monitoring during the cell transplantation procedure in order to potentially correlate the origin of future ventricular arrhythmia to the actual site of injection and reduction in the number of transplanted cells to a maximum of 150 million and limitation of a maximum of 15 injections. The investigators and sponsor implemented these recommendations in full and 9 patients were subsequently included in this particular study.
Description and interpretation of the arrhythmic events observed in this study
Two patients died shortly after their cell transplantation procedure. The first patient (#4003) was a 50 year old man, NYHA class II, presenting with a LVEF of 32% as measured by MRI. He had no history of arrhythmias and was 7 years removed from an anterior wall myocardial infarction. Nine days after an uneventful cellular cardiomyoplasty procedure totalling 288 million cells over 23 injections, the patient died suddenly at home. Prior to this event he complained of dizziness over the telephone, but refused to be admitted as requested by the medical staff. The second patient (#204) was a 58 year old male, NYHA class III presenting with a LVEF of 33% as measured by radionuclide ventriculography. His first myocardial infarction had occurred 7 years earlier and he had also experienced acute myocardial infarction approximately two years earlier which was successfully treated through percutaneous revascularization of the proximal LAD. Because of non-sustained VT runs on holter and inducible VF with electrophysiology testing, an ICD was implanted 2 months before his cell transplantation procedure. Though the injection procedure itself proved uneventful, the patient did experience 2 sustained VT episodes which occurred during NOGA™ mapping and prior to cell injection. These VT episodes were successfully resolved by overpacing and electric cardioversion. A total of 150 million cells were delivered over 15 injections. The holter recording at 9 days post procedure showed multiple sustained and non-sustained VT’s with a rate below the programmed detection zone of the ICD. Three days thereafter the patient was admitted to the hospital because of multiple ICD firings due to an electrical storm, without any evidence of a new ischemic event. In order to control the electrical storm, procainamide was administered which resulted in a worsening of his heart failure.The patient subsequently died 2 days later of cardiogenic shock. Post-mortem examination of the heart showed neither signs of fresh myocardial infarction nor evidence of inflammation or skeletal myoblast engraftment.
In three patients, recruited prior to the implementation of the electrophysiology panel and DSMB recommendations, ventricular tachy-arrhythmia’s occurred, for which ICD’s were subsequently implanted in two. In the first patient (female, 66 years old, NYHA class II, LVEF 26%,#201) symptomatic, multiple non-sustained VT runs were observed during her hospital stay for her 3 month angiographic follow-up procedure. After induction of sustained VT at electrophysiology testing, an ICD was implanted. The second patient (male, 57 years old, NYHA III, LVEF 35%, #4001), was hospitalised due to symptomatic sustained VT 18 days post-injection. Subsequently an ICD was implanted. The third patient (male, 66 years old, NHYA class III, LVEF 28% #102) experienced incidental fast sustained VT runs during the rest ECG at the 1 month follow-up visit. This was successfully treated by amiodarone.
The arrhythmic adverse events encountered in this study have generated a controversial debate over the pathophysiological significance of these events. On one hand it has been suggested that these events could be occurring in the so-called “vulnerable period”, taking place shortly after cell transplantation and possibly related to it, yet others have emphasized the fact that these arrhythmic events are part of the natural history of these high risk patients and are to be expected. However the clustering of these arrhythmic events soon after the procedure should be regarded as worrisome.
It still remains unknown whether the necrosis or apoptosis of the transplanted cells may alone generate arrhythmia or whether true re-entry circuits are be generated through the injection of immature myoblast cells. There is also currently a debate whether the prophylactic use of amiodarone can act as a substitute for the implantation of an ICD in order to safequard against any potential arrhythmogenic phenomena. Although the current study population is at high risk for arrhythmic events due to the natural course of the underlying disease, given the clinical experience (admittedly small) clinical experience with myoblast-mediated cellular cardiomyoplasty and potential risk for initial increase in arrhythmia, the prophylactic administration of amiodarone16,18,19 or ICD placement is recommended until greater (and randomized) clinical experience is gathered. A combination of both amiodarone administration and ICD implantation is not advocated, however, because amiodarone can slow down the ventricular rate below the programmed detection zone of the ICD as well as increase the defibrillation firing threshold for the ICD. Results from other recent studies indicate that the high-risk patients included in our trial would also benefit from ICD therapy20,21. Irrespective of these considerations, more needs to be done to understand the electrical and mechanical integration of the transplanted skeletal myoblasts with the host myocytes so that the safety profile of this therapy is better comprehended.
Functional assessment
Efficacy impact of skeletal myoblast injection on left ventricular dysfunction in chronic heart failure patients was assessed by left ventricular angiography and stress echocardiography in all patients. Left ventricular angiography demonstrated that global left ventricular function remained stable and that no deterioration of these dilated and poorly contracting left ventricles occurred during the 12 months follow-up period. Stress echocardiography showed a descriptive improvement in wall motion score at rest and a trend towards improvement during low dose dobutamine administration after myoblast injection. These results are consistent with the findings of other myoblast studies in which echocardiography was used16,18,19, though the results of this study were somewhat less robust, perhaps due to the relatively low amount of cells injected, the average age of prior infarct (~6 years), or the fact that this study was the first in which skeletal myoblast injections were performed as a stand alone procedure rather than as an adjunct to a revascularization procedure. Unfortunately, the optional use of MRI as a superior assessment method for detecting changes in left ventricular function was limited in this study due to the inclusion of a majority of ICD patients. The small subgroup of non-ICD patients analysed by radionuclide angiography showed that the global left ventricular ejection remained stable through the 6 month follow-up period.
Potential limitations of cell transplantation
At this early stage, cell survival, retention and engraftment following intramyocardial injection is still unknown; survival rates as low as 1-15% have been previously reported22. Furthermore, the dosages used in this study (mean 215 million cells) were relatively low compared with previously published studies16 showing more remarkable improvement –albeit it should be noted that the results of these studies are possibly confounded by concomitant surgical revascularization. Optimal cell dosages still need to be determined. Finally, surviving myotubes may not electrically and mechanically connect with the host cardiomyocytes. Previous investigations have failed to detect a positive staining for connexin-43, the major gap junction protein6,7,14,15,23. Only undifferentiated skeletal myoblasts can express major gap junction proteins, such as N-cadherin and connexin-43. The expression of these proteins is markedly down regulated after differentiation into myotubes, both in culture and after myoblast implantation in normal or cryoinjured hearts23. However one possibility is that electrical coupling can develop through connexins different from connexin-43. Alternatively, it can also be hypothesized that even if the grafted myoblasts remain electrically insulated, they nevertheless respond to the mechanical stimulation exerted by the surrounding myocardium and thus beat synchronously with host cardiomyocytes, though some preclinical studies suggest that this may not be the case24,25. Therefore at this point spectacular improvement in regional and global LV function is not to be expected.
Future concepts
Myoblast-mediated cellular cardiomyoplasty appears to hold significant promise. However to improve the cell transplantation process, and ultimately cardiac function, greater efficiency in the injection procedure and increased cell preservation by genetic or chemical tampering of the cell should be incorporated –monoclonal antibodies capturing injected cells, use of fixative glues (polymers) and transverse rather than of perpendicular injection technique are just some factors which may play a predominant role in the retention of injected cells. To improve survival and electromechanical coupling, a strategy of transplanted myoblasts serving as a platform for gene therapy should be considered; e.g. transplantation of genetically engineered skeletal myoblasts that can secrete HIF1alfa or vascular growth factors like VEGF165 for induction of angiogenesis. Particularly in the hypoperfused ischemic or scarred myocardium this strategy may have a beneficial effect on the survival of the transplanted cells, hence the functional recovery of the injured myocardium26,27. Another promising concept in response to the lack of electromechanical coupling between engrafted myotubes and host myocardium is the transduction of connexin 43 in cultured skeletal myoblasts28 or the combined culture and transplantation of skeletal myoblasts and bone marrow derived stem cells29. These strategies can probably enable a more direct effect of skeletal myoblast transplantation on the failing heart.
In this first multicentre study on percutaneous skeletal myoblast injection as a stand alone procedure in post myocardial infarction heart failure patients we observed a potential increase in arrhythmic events and a trend towards a positive effect on left ventricular function. The statistical and scientific value of these observations may nevertheless be questionable considering the small sample size; therefore larger and randomized studies are needed to address the true safety issue and the efficacy of skeletal myoblast injection in patients with symptomatic severe left ventricular dysfunction after myocardial infarction.
Appendix
Members of the DSMB were: Prof. dr. P.G. Steg, Paris, France, Prof. dr. H.J.J. Wellens, Maastricht, The Netherlands, Prof. dr. J.G..P. Tijssen (chairman), Amsterdam, The Netherlands and Prof. dr. F.W.A. Verheugt, Nijmegen, The Netherlands.
Members of the electro-physiologist panel were: Prof. dr. N. Peters, London, United Kingdom, Prof. dr. L. Jordaens, Rotterdam, The Netherlands.
Acknowledgements
The logistic help during the study by James L. Greene, Richard Spencer and Jack Harvey of the company Bioheart, Weston, Florida, USA and Jeroen Kleijne of Cardialysis B.V., Rotterdam, The Netherlands was gratefully appreciated.

