Introduction
Cardiac failure secondary to ischaemic heart disease is a leading cause of morbidity and mortality1. Infarction leads to cell loss by oxidative stress and reperfusion injury. Chronic ischaemia and myocyte loss produces progressive expansion of the infarct area, fibrous replacement of the myocardium and predisposes to dilation of the left ventricle2, a major factor in survival3. Progenitor/stem cell therapy has potential for promoting structural and functional repair of the myocardium.
Progenitor/stem cells may stimulate either angiogenesis4,5 by the release of growth factors and anti-apoptotic factors (including Akt, VEGF and FGF), and/or vasodilation (by VEGF) with an increase in iNOS bioavailability that may help maintain cell viability and encourage blood flow. More controversially, progenitor/stem cells from the bone marrow, circulating blood or embryonically-derived, may lead to myocardial cell regeneration6,7. However to date, improvements in cardiac function and structure have been modest8,9.
Current delivery techniques include 2-D angiographically guided endocardial injection and catheter based intracoronary release. However, these techniques have general limitations that include imprecise on-table identification of the best areas to target, poor ability in targeting a specific area, and mediocre identification of the treated area at follow-up. In addition, intracoronary release has specific drawbacks such as induction of ischaemia, or the ‘shedding’ of cells into the general circulation. Two particular clinical settings that appear particularly appropriate for progenitor/stem cell therapy are those of recent anterior myocardial infarction that results in reduced LV function and of chronic, ischaemic, dilated cardiomyopathy.
The development and integration of electromechanical mapping technology, NOGA® XP (Biologics Delivery Systems™, Cordis Corporation, Diamond Bar, CA, USA) with the development of a magnetically navigable injection catheter, the MyoStar™ injection catheter (Biologics Delivery Systems™), could transform cell delivery.
This article will review the principles of magnetic navigation with the forthcoming technology; consider how localisation, delivery and follow-up might potentially be improved compared with current techniques; discuss the relevance to two of the clinical settings that might derive the most benefit; and mention the possibilities of improved percutaneous revascularisation that might be used to optimise the local milieu for progenitor/stem cell survival. The purpose of this article is to suggest and speculate upon how this technology might allow integration of the real-time visualisation of target tissue with site directed deliverability in order to reduce procedure times and irradiation, and to simultaneously improve efficacy and follow-up.
Magnetic navigation system
The magnetic navigation system (Niobe®, Stereotaxis, St. Louis, MO, USA) consists of two adjustable permanent magnets on either side of the fluoroscopy table (see Figure 1).
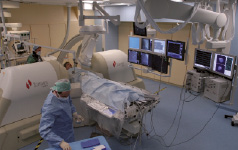
Figure 1. Magnetic navigation system is shown with the magnets in position on either side of the patient.
In essence the system does three things. Firstly, the system operates using a 3-D reconstruction that is either produced from angiographic images (as in the case of coronary arteries) or, alternatively, an imported 3-D reconstruction from an external system e.g. NOGA® XP. Secondly, the spatial orientation and location of this reconstruction is matched to the real-life patient’s internal cardiac anatomy; i.e. coronary artery or cardiac chamber. Thirdly, the model gives the real-time, on-line vectors to direct an external magnetic field to orientate a magnet on an intravascular device to match the direction required for navigation through the 3-D reconstruction. The result is the ability to use 3-D information in real-time in patient therapy.
The magnetic navigation system has been described in detail previously10,11. Briefly, the interacting magnetic field produces a 15 cm uniform magnetic field of 0.08 Tesla that can be increased to 0.1 Tesla. Computer controlled movements of the magnets allow redirection of this externally applied magnetic field vector in 360° in all planes. For cardiac chambers a 3-D volume rendered reconstruction is imported and aligned for navigation. For coronary artery use, the 2-D locations of points on X-ray images are known in relation to the image intensifier, angiography system and table and this allows production of a 3-D reconstruction from 2 views separated by at least 30°. The result is real-time, on-table localisation of the reconstruction within the chest of a patient during a procedure to allow direction of therapy. Adjustment of the magnetic vectors from the model synchronised to the X-ray system adjusts tip-magnet direction in the patient to produce deflection of the wire. This gives reproducibly precise steering of the tip of the intravascular device and this steering is independent of the factors that can restrict conventional procedures such as poor transmission of manipulation.
New technology for progenitor/stem cell delivery
Previous methods for endocardial stem cell delivery had several drawbacks in identification, cell delivery and therapy. Identification of the sites for injections was poor and depended on strategies such as marking acetate sheets overlaid on the X-ray screen, delivery was time-consuming with poor targeting, and follow-up was hampered by imprecise knowledge of where the injections had been and therefore depended on generalised LV measurements rather then the region of interest.
The magnetic navigation system can integrate other 3-D volume rendered information such as MSCT, MRI or the NOGA® XP mapping system, see Figure 2, to give precise steering/direction of injections.
Infarct localisation is possible by current techniques such as MSCT, which is capable of identifying infarcted areas of myocardium12, and integration can provide a 3-D volume rendered map of the damaged myocardium. However this information is not real-time and was of limited use in treating the patient on the table.
New technology is under current development. The NOGA® XP mapping system gives information on the functional and electrical properties of the myocardium simultaneously with real-time, precise localisation by producing a map of the left ventricle (see Figure 2). This may allow differentiation between the extent of the infarct and peri-infarct regions as well as giving valuable information for follow-up. This, together with the MyoStar™ injection catheter to allow magnetically enabled cell delivery (see Figure 3) may not only allow areas to be identified by electrical and mechanical mapping measurements but also deliver injections of cell therapy.
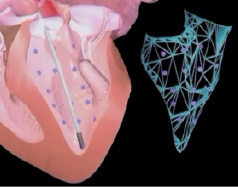
Figure 2. Graphic showing reconstruction of LV mapping.
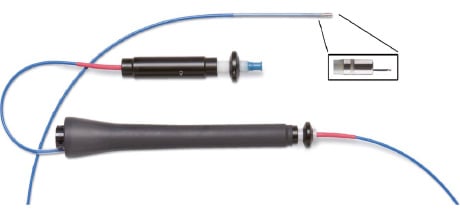
Figure 3. MYOSTAR™ injection catheter to have electromechanical guidance provided by the NOGA® XP.
Potentially, this could give several major advantages. Firstly, integration of real-time electromechanical mapping would give a real-time, on-table 3-D LV map for exact localisation. Secondly, electromechanical mapping may discriminate between viable and non-viable infarcted areas e.g. identifying electrically active but non-contractile areas such as stunned myocardium. Therefore this combination of detection methods could enable new strategies that tailor delivery of cell therapy to particular areas. Thirdly, a magnetically navigable injection catheter could precisely direct cell injection and simultaneously integrate spatial and electrical information. Fourthly, an electromechanical map could aid follow-up both to identify the region on the map that was previously treated, and also to give quantifiable information for specific locations on the map. Definable delivery of stem cells would allow investigation of specific locations or patterns of cell delivery. As different parts of an infarcted area may have discordant electrical and mechanical properties, with intact electrical but poor mechanical activity, this may allow identification of stunned myocardium in penumbral regions that might derive particular benefit from stem cell injection. Additionally, current estimates of the resolution of the new system suggest that this could localise positions to within 1 mm that would represent a major improvement. An idea of the type of the information that may be available with this system for diagnosis and follow-up is seen in Figure 4.
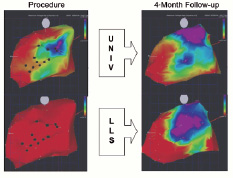
Figure 4. NOGA™ electrical (UNIV, at top) and mechanical (LLS, at bottom) maps from a human patient in the stem cell treatment group. The maps on the left are those performed at the time of injection and maps at right are those at four month follow-up. An area of viability, showing normal electrical activity, can be noted on the upper right map. The maps on the right show improvement in both electrical and mechanical function. LLS = linear local shortening; UNIV = unipolar voltage.
Limitations of current cell delivery techniques
The strategy of endocardial injection has suffered from a number of limitations. The current technique of LV angiography gives mediocre localisation as the entire LV volume is seen only in 2-D pictures from different views. This technique gives little idea of the location of the watershed or penumbral area that might contain stunned rather then necrotic cells. Injection sites, and particularly areas that might be particularly susceptible to improvement with cell therapy, are poorly identifiable in real-time, and are difficult to access with current equipment.
Intracoronary injection has been the other widely used technique and has its own limitations. Release of cells depends on haematogenous spread, and this may be poor in areas that are poorly revascularised or are still occluded, therefore reducing the effectiveness in these areas. This inhomogeneous delivery may prevent delivery to salvageable areas that have competitive flow from a neighbouring territory that is, however, insufficient for long-term survival. As intracoronary injection often uses occlusion of the proximal vessel this results in further ischaemic insult with further, possibly irreversible, damage and cell loss and reduced perfusion pressure leading to collapse of pinched microvasculature. Additionally, loss of cells that do pass through the microvasculature into the general circulation, also known as ‘shedding’, may lead to the drawback of these progenitor cells reaching areas where conditions favourable for angiogenesis are present but clinically unwanted, e.g. neoplastic neovascularisation or ischaemic areas such as retinopathy.
Implications for the clinical situation of cell delivery
Two particular situations may be particularly appropriate for treatment of cell therapy; these are recent anterior infarction with significant LV impairment and non-revascularisable chronic ischaemic cardiomyopathy. While these situations have some similarity such as extensive cell loss and unattractiveness for cardiothoracic surgery, there are specific reasons why precisely directed endocardial injection may hold particular advantages. These have advantages over and above the general advantages of improved diagnosis with two modalities, precise site-directed delivery and follow-up discussed above.
The purpose of using cell therapy in the treatment of recent acute MI is to prevent maladaptation or remodelling and so preserve LV contractile function and thus exercise tolerance. This group may be the most effective group to treat with cell therapy in order to maximise myocardial cell salvage. As discussed above, endocardial injection may be advantageous over intracoronary delivery as it delivers cells to areas that have a restricted or absent blood supply. This direct delivery via the endocardium overcomes the problem of tissue swelling related to ischaemic damage that may cause pinching of the microvasculature to reduce haematogenous delivery to peripheral sections of the infarct territory, i.e. to the penumbral areas that may be particularly suitable for salvage. In addition, endocardial injection decreases shedding of cells into the circulation since cells are injected within the tissue thus placing decreased numbers of cells into the bloodstream.
In the treatment of chronic heart failure there has already been tissue loss and often, this is combined with severe ischaemic coronary disease. Cell therapy aims to reverse the chronic changes by improving the vascular bed and salvaging as much myocardium as possible. This situation would particularly benefit from addition of new myocardial cells, although the ability to produce new myocytes remains controversial. Precise delivery to electrically and mechanically definable areas could allow uniform coverage or other distributions to target specific areas. In addition the risk of perforation of a thin myocardium may be minimised by use of electrical signals via the adjustable and retractable needle providing exact depth control together with monitoring of the ECG for reverse potentials.
Revascularisation
A further option enabled by the magnetic navigation system is the ability to treat complex coronary disease. As the predominant cause of LV dysfunction is ischaemic heart disease, the partial or complete recovery of the native coronary circulation may encourage both recovery of native myocytes and provide a milieu for better uptake and differentiation of stem cells.
The ability of the system to support PCI has been demonstrated in a number of scenarios from simple to complex lesions13 and evidence suggests that this system may be particularly advantageous in more difficult anatomy14 and is capable of successfully treating CTOs15. The integration of MSCT data (see Figure 5) allows the missing segment of the vessel to be judged (see Figure 6) and the superimposition of this data by co-registration gives an indication of the pathway.
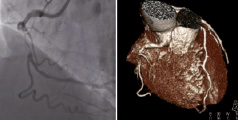
Figure 5. The panel on the left shows the raw data from angiography and the panel on the right shows the reconstructed MSCT.

Figure 6. The panel on the left shows the co-registered points applied to the MSCT image that was imported into Navigant and the panel on the right shows the computer reconstructed pathway that is derived from CT and projected onto the reconstruction in Navigant.
Conclusion
Magnetic navigation may aid the performance of cardiac stem cell transplantation by allowing integration of spatially localised, 3-D volume rendered information with real-time tissue visualisation in multiple modalities to give site directed deliverability to reduce procedure times and irradiation, and improve efficacy simultaneously. The development of the NOGA® XP system and the magnetically navigable MyoStar™ injection catheter may lead to improved detection of non-functional myocardium, better delivery and more precise follow-up.
Acknowledgement
The authors are grateful to Joep Maeijer for his help in the preparation of the images.
References

