Abstract
Aim: Typical intravenous fenoldopam doses result in only minimal increases in renal blood flow and glomerular filtration, while higher doses are associated with systemic hypotension. We hypothesized that local infusion of fenoldopam into the renal arteries would enhance renal perfusion with reduction of arterial hypotension.
Methods and results: Of 20 patients with renal impairment undergoing coronary intervention, 10 received infusion of fenoldopam into the left renal artery (IR). Doses were increased progressively (0.1,0.2,0.4 mcg/kg/min) at 5 min intervals, separated by a 10 min “wash out” period without drug application. Average and maximum peak renal blood flow velocities (APV and MPV) were measured using a flow-wire. A comparison group of 10 patients received renal flow measurements during IV fenoldopam application using the same escalating dose protocol without a drug-free period.
The average peak flow velocity increased from 29.9±10.7 cm/s in the IR group and 25.9±7.7 cm/s in the IV group at baseline (p=ns) to 50.0±13.1 cm/s (IR) versus 31.0±11.9 cm/s (IV) at the maximum dose level (p=0.011). There was a trend towards attenuated reduction of the systemic pressure in the IR group compared to the IV group.
Conclusion: In patients with renal insufficiency, local renal infusion of fenoldopam resulted in an approximately 50% increase in renal artery velocities without substantial blood pressure reduction compared to IV fenoldopam.
Introduction
Low dose fenoldopam (0.05-0.1 mcg/kg/min) infused intravenously (IV) is not effective for the prevention of contrast nephropathy (CONTRAST trial)1. However, the fenoldopam doses selected for the CONTRAST trial have been shown to result in minimal increases in renal blood flow and glomerular filtration in patients with renal insufficiency. An IV dose 4-5 fold higher would likely be more effective, but such doses are associated with systemic hypotension. We hypothesized that local infusion of fenoldopam directly into the renal arteries (IR) would enhance renal perfusion compared to IV infusion and, would produce less systemic hypotension due to a first pass renal clearance.
Methods
Study design and patient characteristics
We conducted an open-label, non-randomized, controlled study to evaluate the hemodynamic impact of local fenoldopam infusion versus systemic fenoldopam application in patients with renal insufficiency and diagnostic cardiac catheterization or coronary interventions. A total of 20 patients with renal insufficiency and scheduled diagnostic coronary catheterization or elective intervention were enrolled in the study. All patients gave their written informed consent prior to enrollment. The first 10 consecutive patients were included in the IR arm (9 diagnostics, 1 intervention), the subsequent 10 consecutive patients were included in the IV arm (8 diagnostics, 2 interventions). Inclusion criteria were renal insufficiency and need for cardiac catheterization. Exclusion criteria were renal artery stenosis, hemodynamic instability, primary PCIs. The study was approved by the local medical ethics committee.
Procedural characteristics
After coronary angiography/intervention, the first cohort received intra-renal (IR) fenoldopam (CORLOPAM, Hospira, Chicago, IL, United States) via a non-occluding JR 4/5 french catheter positioned in the left renal arterial ostium, using the femoral sheath. An escalating infusion protocol increased dose (0.1, 0.2, and 0.4 mcg/kg/min) every 5 minutes, separated by a 10 minute “wash out” period during which no drug was given. Average and maximum peak renal arterial blood flow velocities (APV and MPV, respectively) were measured in the left renal artery using a flow wire (Flowire, Volcano Therapeutics). A comparison cohort received IV fenoldopam infusion, using the same protocol (but without a drug-free “washout” period). Renal flow measurements were performed the same way as in the IR group. Arterial blood pressure measurements were obtained via the femoral artery sheath. Blood pressure values were presented as an average over five measurements.
Study objective
Primary objective of the study was the evaluation of effects on average peak velocity and maximum peak velocity of renal blood flow as well as systemic arterial blood pressure by local versus systemic application of fenoldopam.
Statistical analysis
Data are presented as mean±SD or frequencies. Continuous variables were compared with student t tests. A P value of <0.05 was considered significant. The glomerular filtration rate was calculated using the the Cockcroft-Gault equation.
Results
Baseline characteristics of the study population are listed in Table 1.
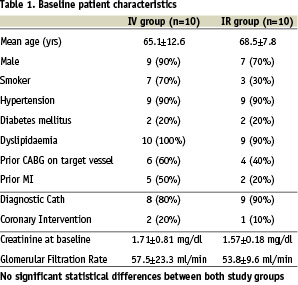
There were no significant differences between the baseline parameters. All patients had renal insufficiency with creatinine levels above the upper limit of normal (>1.2 mg/dl). The average creatinine levels were 1.71±0.81 mg/dl in the IV group and 1.57±0.18 mg/gl in the IR group (p=0.598). The average GFR were 57.5±23.3 ml/min (IV) and 53.8±9.6 ml/min (IR), respectively (p=0.66). There was a dose-related increase in both the renal artery APV and MPV following IR, but not IV fenoldopam (Chart 1 and 2).
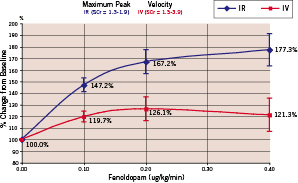
Chart 1. Maximum peak velocity of renal blood flow
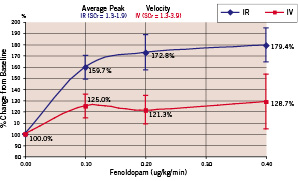
Chart 2. Average peak velocity of renal blood flow
Intra-renal fenoldopam produced significantly greater peak flow velocities compared to IV Fenoldopam (Table 2, Chart 1 and 2).
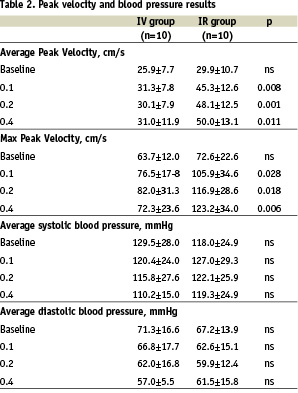
The average peak flow velocity increased from 29.9±10.7 cm/s in the IR group and 25.9±7.7 cm/s in the IV group at baseline (p=ns) to 50.0±13.1 cm/s (IR) versus 31.0±11.9 cm/s (IV) at the maximum dose level (p=0.011). Typical examples of IR and IV flow results are shown in Figures 1 and 2.
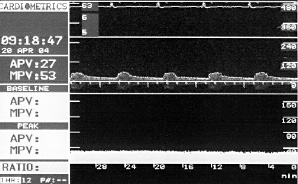
Figure 1. Example of flow velocity measurements in a patient with IV application of fenoldopam. No evidence for a significant dose-dependent impact on renal flow velocity.
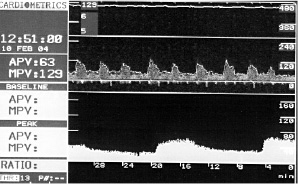
Figure 2. Example of flow velocity measurements in a patient with IR application of fenoldopam. Obvious dose-dependent impact on renal flow velocity.
While systemic systolic and diastolic arterial pressures showed a clear trend to decrease in a dose-dependent manner following IV fenoldopam treatment, arterial pressure remained almost unchanged during IR delivery (Table 2). At baseline, the average systolic/diastolic pressure was 129.5±28.0 mmHg and 71.3±16.6 mmHg in the IV group and 118.0±24.9 mmHg and 67.2±13.9 mmHg in the IR group. At the high dose level, average systolic/diastolic pressure was 110.2±15.9 mmHg and 57.0±5.5 mmHg in the IV group versus 119.3±24.9 mmHg and 61.5±15.8 mmHg in the IR group. However, there were no significant differences in the arterial pressures. There were no procedure-related complications observed.
Discussion
Fenoldopam is a selective agonist of dopamine-1 receptors that causes a dose-dependent increase in renal blood flow and blunts the reduction of glomerular filtration rate (GFR) and renal blood flow that occurs in animal models of radiocontrast nephropathy2. It increases blood flow (and therefore oxygen delivery) to the renal medulla3 which is most vulnerable to renal ischemia and may also reduce renal metabolic demand by inhibiting sodium reabsorbtion4. Fenoldopam’s activity profile therefore, appears well suited for prevention of contrast nephropathy.
While several initial studies suggested that systemic fenoldopam application was effective at both reducing radiocontrast nephropathy as well as decreasing the need for dialysis in high risk patients (creatinine > 1.5 mg/dL)5,6 undergoing contrast injection, results of randomized controlled trials have been more controversial1,7. Because fenoldopam has dose-dependent effects on renal blood flow (from 0 to 0.5 mcg/kg/min)8,9 higher doses of fenoldopam may be more effective in attenuating increases in creatinine following contrast injection. However, the maximal fenoldopam dose is typically limited to 0.1-0.2 mcg.kg/min by progressive systemic arterial hypotension above this ceiling.
We reasoned that intra-renal infusion would allow exposure of the kidney to higher blood levels, since the drug would be diluted less in the 20% of cardiac output that supplies the kidneys (10% to each kidney) than it would be by infusion into the systemic circulation. Our study confirms that local delivery of fenoldopam into the renal artery in patients with renal insufficiency enhances renal flow velocity and might reduce systemic arterial hypotension compared to IV delivery at the same infusion rates. Our observation confirms that even higher infusion rates (including 0.4 mcg/kg/min) are well tolerated, without systemic arterial hypotension, when delivered locally. Thus, the results of this study suggest the existence of a first-pass clearance of infused fenoldopam by the kidney. The combination of greater local renal arterial drug concentration at the usual IV doses, plus the ability to tolerate higher absolute doses (up to 0.4 mcg/kg/min) with IR fenoldopam infusion, combine to maximize renal flow dynamics to a degree that was not possible with traditional intravenous infusions. In this context, the failure of the CONTRAST trial may have been due to inadequate dosing of fenoldopam, which could be overcome by using the intra-renal infusion route.
Effective therapy of this type would require a device that could perform simultaneous left and right intra-renal infusion of fenoldopam (or other vasoactive drug) without preventing the performance of the diagnostic or therapeutic cardiac intervention for which the contrast was being administered. Such a device has been recently developed (Benephit catheter, FlowMedica), and has been FDA 510-K cleared for use in clinical research as investigational infusion catheter. Preliminary data suggest that this device can be placed easily and simultaneously in both renal arteries. Given these experiences, it may be appropriate to revisit local intra-renal fenoldopam delivery as a strategy for maximizing renal hemodynamic effects and minimizing systemic arterial hypotension, for the prevention or treatment of contrast induced nephropathy. A pivotal randomized trial of this therapeutic approach is currently underway.
Limitations
This study was non-randomized and included only a small patient population. Larger controlled studies are necessary to prove the efficacy of this treatment concept. Due to the study design, there was no information available of the hemodynamics of the contralateral renal artery.
Conclusion
In patients with renal insufficiency, local renal infusion of fenoldopam has the potential to increase renal artery flow velocity without substantial blood pressure reduction compared to intravenous fenoldopam application.

