Abstract
Valvular aortic stenosis is a frequent disease, especially in the elderly, and is associated with increased morbidity and mortality in symptomatic patients. Significant progress has been made in the field of percutaneous valve implantation, a technique that is currently applied exclusively to treat high-risk patients, who cannot undergo open-heart surgery. For the time being, two different types of aortic valve prostheses are available, balloon-expandable stent valves and self-expanding stent valves. Originally, percutaneous aortic valve implantation was performed via the antegrade approach with two main limitations, the transseptal puncture and the advancement of the prosthesis through the mitral valve. Pericardial tamponade has been associated with transseptal punctures. We and others have reported mitral valve regurgitation post valve implantation due to mitral leaflet tear induced by the guidewire used for the antegrade approach. Recently, the retrograde implantation of aortic valve prosthesis has been proposed as an alternative route in order to overcome the aforementioned disadvantages. The accurate positioning of the aortic prosthesis is essential and is difficult to achieve during left ventricular contraction. Rapid ventricular pacing or extracorporeal circulation have been successfully used to temporary reduce the exerted forces throughout systole. In the present manuscript, we report the employment of TandemHeart™ support during the implantation of the aortic CoreValve™ prosthesis in a pig. The procedure was performed retrograde under intracardiac echocardiographic guidance.
Introduction
Valvular aortic stenosis is a common disorder and the incidence is rising with the aging of the population in the western countries. The prevalence in individuals older than 65 years ranges between 2-7%1. Although, surgical valve replacement is considered the standard treatment for severe aortic valve stenosis, it still remains a major, highly invasive procedure with all the concomitant risks associated with general anaesthesia, sternotomy and cardiopulmonary bypass. Consequently, surgical mortality averages 2-9% and is higher in patients with comorbid conditions2. Percutaneous aortic valve replacement has been proposed as an alternative treatment for severe aortic valve stenosis, but at present is mainly restricted to patients being considered at high surgical risk3. Percutaneous valve replacement will offer symptomatic relief and potentially also longer survival
The aortic valve prosthesis is mounted on a stent made form stainless steel or nitinol. The stent is either balloon-expanded or self-expanding. The balloon-expanded valves that have been successfully implanted in humans include the Paniagua Heart Valve (Endoluminal Technology research, Miami, USA)4, and the Cribier-Edwards Valve (Edwards Lifesciences Corporation, USA)5. The CoreValve™ is a novel bioprosthetic valve pre-mounted and sutured on a self-expanding nitinol stent (Figure 1)6.

Figure 1. The proximal part of the stent is fastened in the ascending aorta (arrow) and the distal part, which has a high radial strain, pushes aside the calcified leaflets, preventing recoil (arrowhead). The bioprosthetic valve is located at the middle part and is constructed to circumvent the coronaries.
The valve is made of a single layer of porcine pericardial tissue constructed in a trileaflet configuration. During the placement of the device, the proximal part of the stent is anchored in the ascending aorta with the distal part, which has a high radial strain, pushing aside the calcified leaflets, preventing recoil. The bioprosthetic valve is located at the middle part and is constructed to secure access to the coronaries. The CoreValve™ is delivered via a 21F catheter, which incorporates distally the stent.
In the current article, we describe aortic valve replacement in a pig with the use of the TandemHeart™ (Cardiac-Assist, Inc.; Pittsburgh, Pa) cardiac assist device, guided by intracardiac echocardiography imaging with the AcuNav™ catheter (Siemens AG, Germany). The procedure was carried out according to a protocol approved by the Committee on Experimental Animals of the Erasmus University.
Case report
A Yorkshire, landrace, duroc pig of 62 kg underwent percutaneous aortic valve implantation. After an overnight fast, the animal was sedated with 20 mg/kg ketamine hydrochloride and 0.5mg/kg midazolam and anaesthetized with sodium pentobarbital (15 mg/kg i.v.). Following endotracheal intubation, the pig was connected to a ventilator that administered a mixture of oxygen and nitrogen (1:2, v/v). Anaesthesia was maintained with pentobarbital 10-20 mg/kg.h–1. Cardiac and respiratory functions were monitored throughout the procedure.
Initially, a 12 F sheath was inserted in the right iliac vein and the AcuNav™ catheter was positioned in the right atrium. Thereafter, the outflow cannula of the TandemHeart™ was inserted surgically into the abdominal aorta followed by the interatrial septum puncture (Figure 2A), which was performed under direct intracardiac echocardiographic visualization. Subsequently, the inflow cannula was advanced into the left atrium (Figure 2B).
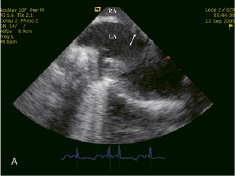
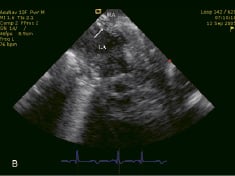
Figure 2. (A) Inter-atrial septum puncture under intracardiac echocardiographic guidance. (B) Inflow cannula of the Tandemheart™ assist device into the left atrium (arrow). RA: right atrium, LA: left atrium.
Heparin sodium 5,000IU was administered immediately after the transseptal puncture. From then on, the procedure was performed under continuous cardiac assist device support (Figure 3A and 3B).
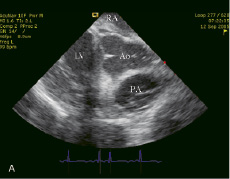
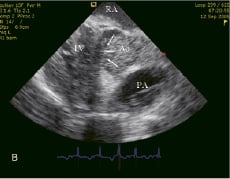
Figure 3. (A) TandemHeart™ off. (B) TandemHeart™ on. Spontaneous contrast is observed in the aorta. The aortic valve leaflets are immobilized (arrows). Ao: ascending aorta, RA: right atrium, LV: left ventricle, PA: pulmonary artery.
The 21F catheter that carries the bioprosthetic valve was also surgically delivered via the left carotid artery. After crossing the aortic valve using a 0.035 guidewire, the delivery catheter was gently inserted and advanced through the native aortic valve. The external sheath of the delivery catheter was thereafter slowly pulled back and the valved stent expanded under fluoroscopic and echocardiographic guidance (Figure 4).
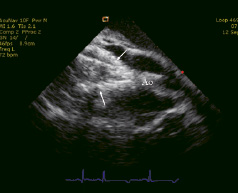
Figure 4. Expansion of the valved stent (arrows) under echocardiographic guidance. Ao: ascending aorta.
Finally, the delivery system was then removed and intracardiac echocardiographic control confirmed the appropriate location of the prosthesis (Figure 5A and 5B) and verified the good function of the implanted valve. At the end of the procedure the animal was sacrificed and the exact position of the bioprosthetic valve was demonstrated (Figure 6).
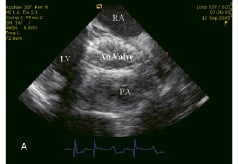
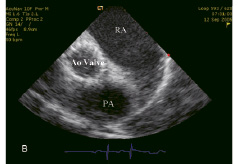
Figure 5. Echocardiographic control of the implanted Corevalve™ prosthesis. (A) Long axis of the valve. (B) Short axis of the valve. RA: right atrium, LV: left ventricle, Ao valve: Aortic valve prosthesis, PA: pulmonary artery.
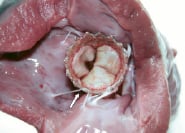
Figure 6. Post-mortem examination: view from the left ventricle showing that the endoventricular struts is partially entangled (arrow) in the chordae of the mitral apparatus as the infra-annulus region is very short in this type of animal model.
Discussion
The implantation of a self-expanding aortic prosthesis has been previously evaluated in animals6,7. The experience in the clinical setting is limited8,9.
Initially, the aortic valve prosthesis was inserted antegrade via the venous circulation, was advanced after transseptal puncture into the left atrium and was finally delivered through the mitral valve. The antegrade approach has been proven to be feasible and safe but is technically more complicated and has been associated with increased risk for pericardial tamponade, atrial and ventricular arrhythmias, mitral valve injury and hemodynamic instability10. Recently, the retrograde approach has been proposed as an alternative route in order to overcome the aforementioned disadvantages. However, even if the retrograde approach is less demanding technically, it also has its drawbacks, such as the insertion of large sheaths in the femoral artery and the advancement of bulky catheters through potentially torturous femoral and iliac arteries, the aortic arch and the stenotic aortic valve.
The accurate positioning of the aortic valve prosthesis is essential and is difficult to be achieved during left ventricular systole. Rapid ventricular pacing at 200bpm is typically used during placement of the prosthesis across the aortic valve in order to reduce cardiac output and minimize pulsatile transaortic flow and thereby preventing dislocation of the device5. However, rapid ventricular pacing acutely increases oxygen consumption and pulmonary wedge pressures11. Additionally, throughout the placement of the prosthesis the circulation is momentarily obstructed, resulting in decreased blood supply to the peripheral and the coronary arteries. The use of ExtraCorporeal Membrane Oxygenation (ECMO) devices could provide adequate blood supply simultaneously immobilizing the native aortic valve. ECMO has been used in the past for patients undergoing high-risk angioplasty. Their major limitations are the increased peripheral vascular complications, the higher administered heparin doses and the potential microembolization of particles from the membrane oxygenator. Moreover the presence of a perfusionist is mandatory12. Haemodynamic support with the use of the TandemHeart™ during percutaneous aortic valve replacement provides adequate coronary and peripheral perfusion, is safe and feasible and offers several advantages:13 (I) It stabilizes severely decompensated patients who are at increased risk for adverse cardiac events. (II) Cardiac assist effectively diminishes the ventricular ejection fraction and abolishes the haemodynamic forces exerted throughout the placement of the bioprosthetic valve, facilitating the positioning and avoiding the need for pacing. Simultaneously it provides circulatory support and unloads the left ventricle, allowing gradual recovery of the ventricular function after the placement of the bioprosthetic aortic valve. (III) The TandemHeart™ may serve as bridge to emergency surgical valve replacement in case this is required during the percutaneous procedure. (IV) Further, it supports and stabilizes the patient in the course of the post-treatment period, enabling swift recovery. The main disadvantage of the TandemHeart™ is the need for transseptal punctures, the current size of the cannula (21F for the left atrium and 15 or 17F for the femoral artery) and the fact that the adequacy of the device functioning is critically dependent on the right ventricular performance.
Intracardiac echocardiography provided excellent guidance during the transseptal puncture and the implantation of the aortic valve prosthesis. Typically, the exact positioning of the prosthetic valve in healthy animals mandates a supra-aortic angiogram to delineate the aortic silhouette and the aortic valve orifice. However, in our case, this was not necessary, since intracardiac echocardiographic imaging with the Acunav™ catheter allowed accurate placement of the prosthesis with direct visualization of the native valve.
In the future, decompensated patients with severe aortic valve stenosis and high surgical risk may benefit from the percutaneous aortic valve implantation. The retrograde technique in combination with cardiac assist support and intracardiac imaging is a promising approach that merits further investigation.

