Description
Implant device and delivery system.
The Ventricular Partitioning Device (VPD) is an intraventricular Implant that is deployed in the Left Ventricle (LV) in patients with regional wall motion abnormalities following myocardial infarction (MI). The implant device is delivered via a guiding catheter and delivery system introduced percutaneously from the femoral artery by the standard techniques for left heart catheterization.
The implant device is an ePTFE membrane bonded to an expanded NiTi frame in the configuration of an inverted umbrella. (Figure 1)
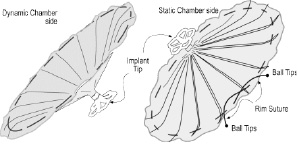
Figure 1. VPD device diagram.
The stem of the umbrella is positioned in the apex of the LV and has a collapsible foot designed to hold the implant off the endocardium a preset distance. The struts that support the membrane have a parabolic configuration that allows the rim of the membrane and the passive anchors located at the tip of each of the struts to engage the endocardium and maintain the position of the Implant by spring tension against the endocardial wall (Figure 2).
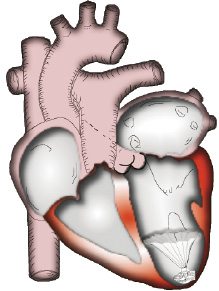
Figure 2. Implant positioned in LV Apex.
The Guide catheter is a braided catheter with a preformed distal tip that is designed to orient it directly into the LV apex. A Dilator/introducer is placed within the guide catheter during placement, and accommodates standard intravascular guidewires to assist in placement of the guide catheter. The guide catheter with dilator in place is advanced retrograde across the aortic valve with the guidewire positioned beyond the tip and is placed at the apex of the LV. The guide catheter has a side port and haemostatic valve that allows injection of contrast around the delivery catheter.
The delivery catheter is used to advance the VPD Implant within the guide catheter following placement of the guide catheter in the LV. It has a distal screw mechanism for engaging and disengaging the Implant. The delivery catheter has a compliant balloon located just proximal to the screw connector that allows for expansion of the VPD membrane after being advanced beyond the guide catheter during deployment in the LV (Figure 3).
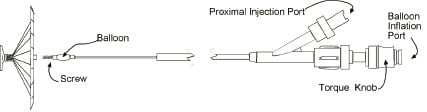
Figure 3. Delivery Catheter and Implant.
Once the guide catheter is positioned as described above, the placement of the VPD Implant proceeds as follows. The introducer/dilator is removed and the delivery catheter with the implant attached is advanced through the guide catheter to the distal end (Figure 4).
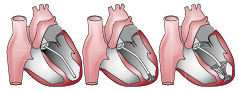
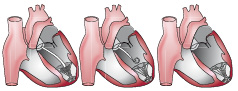
Figure 4. Placement of the VPD implant device in the LV.
The expansile foot is exposed beyond the guide catheter and placed against the endocardium. The guide catheter is retracted while the delivery catheter is held in place until the membrane component exits the guide catheter. At this time the balloon is inflated for 30 seconds seating the passive anchors at the rim of the membrane in place in the endocardial wall at the level of the ventricle just below the papillary muscles. Sizing is accomplished by measuring the cross-section of the ventricular chamber three and a half cm proximal to the apex on the ventriculogram prior to placement, and over sizing the device between 30 and 50% of that dimension. The balloon is then deflated and the delivery device disengaged and removed from the guide catheter. At this point all catheters are removed from the arterial system and the puncture sites addressed.
History
Drs. Branislav Radovencevic MD and Serjan Nikolic PhD conceived of the ventricular partitioning approach in 1999, and evaluated some early prototypes surgically implanted in a calf model. The goal was to decrease end diastolic volume thereby decreasing myocardial work and wall stress that has now been shown to have an immediate haemodynamic benefit. Drs. Radovancevic and Nikolic considered how the surgical endoventriculorrhaphy and aneurysectomy procedures effectively improved LV function in large dilated hearts, and how a percutaneous catheter mediated intervention tailoring the ventricular geometry without the need for a thoracotomy which could benefit patients with the appropriate anatomical substrate.
A secondary effect of ventricular partitioning and perhaps of greatest long-term patient benefit is the exclusion of the infarcted anterior wall that is most vulnerable to dilation and thinning. Regardless of whether infarction produces transmural or non-transmural necrosis, the associated dyskinesis or akinesis can result in progressive remodelling of the infarcted and subsequently the non-infarcted regions of the myocardium.
Technical specifications
The Implant is currently available in two sizes: 75mm and 85 mm. These have been found to be the most appropriate sizes for the target patient population. The guide catheter and delivery system are 14 Fr. and come in two different tip configurations.
Indications for use
The VPD Implant is intended for use in patients following myocardial infarction to prevent the deterioration of LV function from progressive ventricular remodelling. Remodelling leads to increasing abnormal wall motion, myocardial thinning and an elongation of the affected region. Infarct expansion and wall thinning are mainly the result of muscle fibre slippage. Geometrical changes of the infarcted area and the loss of myocytes increases wall stress within the region as well as at the border zone between the infarct and the normal myocardium. These early events during an acute MI initiate the subsequent cascade of changes leading to progressive LV enlargement.
Patient selection involves identifying post LAD infarct patients, without ongoing ischaemia, that have demonstrated remodelling and regional wall motion abnormalities, and who have not progressed to extensive global dilation and significant deterioration of the function of the mitral valve.
Tips and tricks for delivery
The technique is accomplished easily by anyone with experience in catheterization of the left heart. Accessing the LV apex is simple and the procedure need not take longer than 10 minutes once the access sheaths are in place and the device prepped. The more technically demanding part of the procedure is the preparation of the implant prior to insertion. The implant must be collapsed and inserted into the guide catheter following attachment to the delivery catheter while keeping all three components submerged in heparinized saline to eliminate air from the system. Detailed instructions are provided in the Instructions for Use. It is suggested that the user practice this procedure before performing their first case.
Other issues to be aware of are the measures to ensure that embolic events are controlled. It is recommended that patients be placed on Coumadin for three months and an ongoing regimen of clopidogrel and aspirin should be considered. In addition to the issue of clot formation on the implanted device is the issue of the patient with a mural thrombus as is commonly seen in patients with wall motion abnormalities and ventricular remodelling. Adequate pre-procedural evaluations must be performed to identify these patients so they can be excluded from consideration.
Pre-clinical and clinical experience
Over 150 animals were studied during the development of the VPD device. Development of the ovine infarct model was pursued in parallel with the evaluation of early prototypes. The final phase of the sheep study following design freeze confirmed the early indications of haemodynamic benefit, the safety and utility of the final design, and its readiness for clinical investigation. The final 30 animals were studied under GLP conditions. That series included subjects survived for varying increments up to 12 months and included angiographic and haemodynamic assessments as well as complete histological examinations. Figure 5 shows a pressure volume study performed showing a normalization of the pressure/volume relationship in the implanted animals compared to the untreated state.
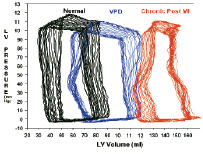
Figure 5. Pressure Volume Relationships Normal (black loops - pre-infarct), Chronic State (red loops - post Infarct) and post VPD implantation (in blue - chronic state).
Though the number of study subjects is small, there are enough to show statistical significance evidencing improvement in haemodynamics.
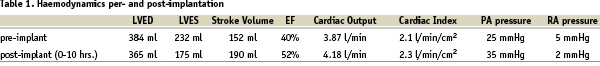
Once sufficient experience in animals was gained, two patients, both candidates for endoventriculorrhaphy were studied on the day of their surgery at the Dedinje Cardiovascular Centre in Belgrade, Serbia. The VPD device was implanted in the cath lab via the percutaneous femoral approach following baseline angiograms and haemodynamic assessments. Those studies were repeated within one hour following implantation. The patients were then taken to the O.R. and the anticipated surgical procedure was performed during which the VPD Implants were removed and examined. No untoward effects were noted in either patient and acute improvements in LV function were noted after deployment of the device, as measured by increases in cardiac output, stroke volume and ejection fraction and a decrease in LVESV and LVEDV. Figure 6 shows a representative comparison of pre- and post- implantation and the improvement in LV function as evidenced by the improvement in ejection fraction.
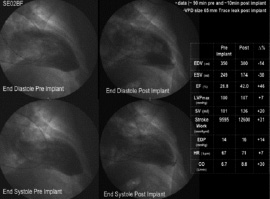
Figure 6. Angiograms and haemodynamic values for a representative patient from the early series in Belgrade.
An ongoing series of chronic implants being conducted in Europe at several centres is demonstrating very encouraging results. The interim results of this series will be reported at the EuroPRC meeting, along with more detailed descriptions of the use of the device and the clinical results.