Introduction
Coronary stenting has markedly improved the safety of percutaneous treatment of coronary artery disease by scaffolding the atherosclerotic plaque, treating the dissection following balloon inflation, preventing the elastic recoil and the negative remodelling. Restenosis, however, remains a major limitation. The advent of the first generation drug eluting stents (DES) has undoubtedly reduced the incidence of restenosis but the recent emergence of data indicating an increased risk of late stent thrombosis with inevitable risk of myocardial infarction (MI) and death compared with the bare metal stent (BMS), has highlighted the need for further investigation about the safety of DES1,2. Drugs coated on the currently available DES, create a delayed healing response and inhibit endothelial cell growth aggressively as a primary modus of reducing neointimal formation3. Duration of dual antiplatelet therapy (DAPT) is another important factor. Premature cessation of DAPT often causes stent thrombosis.4-6 Hence a stent coated with a drug like Trapidil (Triazolopyramidine) with unique and multiple modes of action to reduce neointimal proliferation, yet not interfering endothelial healing, may provide a favourable and predictable balance between safety and efficacy.
Trapidil
Trapidil was initially developed in 1971 as a vasodilator and anti-anginal agent and later also used as antiplatelet therapy. However, over the past few decades, studies have found Trapidil to be a potent inhibitor of platelet aggregation and activation, vascular smooth muscle cell proliferation, and monocyte/macrophage migration and activation, thereby making it a potentially potent anti-restenotic agent. Trapidil is a water and alcohol soluble drug. The lipophilic nature of the drug allows it to permeate cell membranes rapidly to induce its molecular and cellular effects. The structural formula of Trapidil is given in Figure 1.
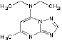
Figure 1. Chemical structure of Trapidil.
The biological actions of Trapidil are wide ranging. Trapidil’s role as a vasodilator is achieved by competitive inhibition of phosphodiesterase1-4. Additionally, the drug decreases platelet aggregation, which is attributed to its ability to increase prostaglandin synthesis7 and inhibits platelet activation by reducing thromboxane synthetase.8,9 Trapidil has also been shown to inhibit the proliferative effects of platelet derived growth factor (PDGF) on vascular smooth muscle cells via activation of protein kinase A (PKA), which blocks the effects of mitogen activating protein (MAP) kinase10,11 enabling Trapidil to inhibit neointimal hyperplasia. In addition, it inhibits the release of monocyte chemoattractant protein –1 (MCP-1), thereby preventing the accumulation of macrophages following balloon arterial injury.12 Further studies demonstrate that Trapidil also inhibits macrophage activation through the CD40 pathway13. Matrix metalloproteinase2, a marker of inflammation is also suppressed by Trapidil through its effect on CD 40 ligand in human abdominal aortic cell culture.14 Trapidil thus acts as a vasodilator while inhibiting platelet aggregation, platelet activation, vascular smooth muscle cell proliferation, and monocyte/macrophage infiltration and activation. It also reduces interleukin (IL) 1-6 and IL-12 production. It has thus potent anti-inflammatory effect as well (Figure 2).
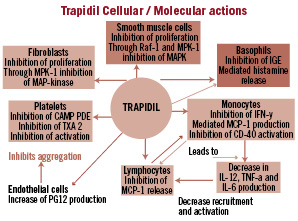
Figure 2. Showing multiple modes and sites of action of Trapidil.
Animal studies
Trapidil has been tested in several animal models to further verify the above outlined effects. In an ischaemic heart model, Trapidil was shown to inhibit the effects of thromboxane A2 by inhibiting platelet aggregation while reducing ischaemic changes in ECG and elevating serum high density lipoproteins (HDL)9. In a rat aorta balloon denudation model, Trapidil reduced smooth muscle cell (SMC) proliferation and neointimal hyperplasia without affecting the extent of endothelial regrowth.15 Trapidil protects ischaemic heart from reperfusion injury by stimulating PKA II activity.16
Bonisch et al17 demonstrated direct stimulation of Protein Kinase-A (PKA) resulting in an inhibition of Raf-1/ MAP kinase thereby inhibiting SMC proliferation induced by vessel injury. Animal studies in a variety of balloon injury models have repeatedly shown the efficacy of Trapidil in the prevention of neointimal hyperplasia. This antiproliferative effect of Trapidil was demonstrated in dose response manner in the rabbit iliac balloon injury model with decreased restenotic rates. 18,19
Human oral use
In humans, Trapidil has high bioavailability after oral dose with a half-life of 2.4±1.1 hours.20 Hepatic excretion is low and no renal clearance occurs.21
Reported side-effects including occasional GI intolerance, headache and dizziness were not different from those of aspirin.22 Trapidil’s effect on haemodynamics, coronary circulation and myocardial metabolism were very favourable. In 19 patients (10 with chest pain syndrome and nine with coronary heart disease), coronary dilation and an increase in cardiac output without a corresponding increase in myocardial oxygen consumption were noted.23 Furthermore, Kurita et al24 reported beneficial systemic haemodynamics in patients with depressed ventricular function following administration of Trapidil. Very high efficacy and tolerability of oral Trapidil was noted as therapy for angina in two large scale double blind trials.25,26 A study of long term use of Trapidil in patients with documented coronary artery disease showed improved prognosis and significant reduction of event rates in Trapidil patients Hirayama et al.27
Oral Trapidil use; studies to reduce restenosis following PCI
There have been several clinical studies focusing on the effects of oral Trapidil on restenosis following percutaneous coronary intervention22,28-32. A randomised comparison of Trapidil versus aspirin showed a reduction, though non-significant, in restenosis in Trapidil group compared to aspirin following Palmaz-Schatz stent implantation in chronic total occlusion at six months follow-up.22 In another randomised trial, Okamoto, et al28 treated patients with placebo (n=34) or 200 mg of oral Trapidil (n=30) from seven days before percutaneous transluminal coronary angioplasty (PCTA) up to six months following and found lower rates of binary restenosis in treated patients (20% vs. 44.1%, p< 0.05).
In a randomised trial by Nishikawa, et al29, a strategy of oral Trapidil (300 mg TID) plus aspirin (300 mg daily) was compared with aspirin (300 mg daily) plus Dipyridamole (50 mg TID) from day two to four months following, PCTA (80 patients in each group). Binary restenosis was lower in the Trapidil group as compared to the Dipyridamole group (20% vs. 38%, p < 0.05). This equates to a 47% reduction versus the control arm, similar to the Okamoto publication described above.
In the randomised double blind multicentre STARC study30, patients were treated with either aspirin 100 mg TID or Trapidil 100 mg TID orally, for six months after PCTA. The Trapidil group (n=128) had a lower rate of restenosis (24% vs. 39.7%, p<.01) as compared to the control arm (n=126). This equates to a 40% reduction versus the control arm, measuring closely to that of the Okamoto and Nishikawa studies mentioned above. In STARC II trial31, however this beneficial effect of Trapidil was noted in the balloon angioplasty group only, but not in patients with stent implantation.
Clinical events at follow-up were similar in the two groups except that recurrent angina was significantly more frequent in the aspirin group: 43.7 versus 25.8 in the trapidil group (p<0.01). The TRAPIST study (Serruys et al),32 a multicentre randomised placebo controlled trial, studied the efficacy of Trapidil for the prevention of restenosis after coronary stenting with WallStent™ implantation. A total of 303 patients (148 Trapidil, 155 placebo) underwent WallStent™ implantation, and 139 patients in the placebo group (90%) and 138 in the Trapidil group (93%) had repeat catheterisation at 26±2 weeks. There were no significant differences between Trapidil and control groups in terms of neointimal volumes, percentage obstruction volumes, angiographic minimal luminal diameters and binary restenosis rates, cumulative incidences of major adverse cardiac events or anginal complaints. Previous studies focused on lesion lengths of < 15 mm, while the TRAPIST study treated lesions > 25 mm in length. Therefore, it is possible that the lesion length and insufficient amount of drug at target site might have been mitigating factors leading to the differences observed. However, despite the neutral result of Trapidil in this study and in the stent arm of STARC II, all these studies have demonstrated its safety and lack of side-effects or adverse interaction with concomitant anti-angina therapy. There is thus a sufficient reason to study Trapidil as an agent coated on a stent in the prevention of restenosis.33
In addition, the case to investigate the use of Trapidil in a drug eluting stent is further strengthened because of its wide ranging mode of action. The delivery and concentration of the drug at the target site while coated on a stent is far more than what can be achieved by oral therapy. It may not only prevent neointimal proliferation but, due to its unique mode of action, also reduce the need of prolonged dual anti-platelet therapy. Trapidil can be effectively incorporated into a drug /polymer coating on a stent for sustained release at the target site.34
Device description
The INTREPIDE™ Trapidil-eluting coronary stent system consists of a balloon expandable stent coated with a layer of Trapidil and Parylene C. The Trapidil-eluting stent is pre-mounted on Nimbus™ balloon (ClearStream, Enniscorthy Co. Wexford, Ireland) which is currently used as a PTCA Catheter. The controlled release of Trapidil is achieved through a barrier of parylene (described below) to deliver the drug to the target lesion.
The stainless steel stent
The stent is laser cut from a 316L stainless steel tube and electro polished following completion. Figure 3 depicts the physical design of the stainless steel stent.

Figure 3. Physical design of the stainless steel stent.
The stent contains ultra-thin rounded struts with an area of 0.56 mm2 between each strut end. The stent features repeated consistency in cell sizes alternating between expansion struts and bridges throughout the length of the stent. The expansion struts are 0.079±0.018 mm in diameter while the bridges are 0.089±0.018 mm in diameter. Upon expansion, the stent foreshortens by 0.21 mm at a 3.0 mm diameter, by 0.45 mm at a 3.5 mm diameter, and by 0.46 mm at a 4.0 mm diameter.
The bare metal stent is plasma etched to ensure optimum reproducibility of drug coating. An ultrasonic spray coating process is then used to apply approx 7 µg/mm2 layer of Trapidil, dissolved in 100% ethanol, onto the stent surface. This method of application results in reproducible and optimum amount of the drug coating.
Polymer component
The Intrepide™ coronary stent utilises Parylene C as the barrier coating to control the release of Trapidil. Parylene C is extremely stable chemically and biologically. It is derived from di-paralyxylene, a monomer gas, by a special thermal process and the polymerisation process does not involve any solvents, catalysts or other additives thereby eliminating possible contamination in the final coating. A single 3.5 µm layer of Parylene C is coated over the drug as a barrier coating to control the release of the drug. During the Parylene deposition, the stent remains at room temperature, preventing the possibility of any temperature variations effecting drug’s physical and chemical characteristics. Parylene is widely used in medical device coatings, including vascular catheters to improve lubricity to reduce the friction of insertion or removal of guidewires and is considered an ideal coating for several implantable devices including pacemaker and ICD leads,35 due to its electrical insulation properties.36 In addition to the coating characteristics, Parylene surfaces have also shown to be biocompatible,37,38 manifesting extremely low thrombogenic or even anti-thrombogenic properties. Parylene coated tantalum stents in a swine model showed reduced platelet accumulation.39
A small amount of Parylene is utilised in the coating process of many currently available drug eluting stents. The parylene coating meets the requirements of USP 26, NF 21, 2003 for Class VI Plastics-70°C according to tests performed by Toxicon Corp. (Bedford, MA, USA).
Stent delivery system
The Nimbus™ balloon catheter is the delivery system. It is available as a rapid exchange PTCA and stent delivery catheter utilising XxtraFlex™ technology with enhanced pushability and flexibility. It consists of a semi-compliant balloon and has approximately a 10% increase in diameter from nominal to rated burst pressure. It features refold memory with rewrap properties. Platinum radiopaque marker bands are used to define the stent mounting and to locate the stent under fluoroscopy. The rated burst pressure is 16 atm and the average burst pressure is 22 atm for 2.5 -4.0 balloon (20 atm for 4.0 mm x 30 mm balloons).
Vascular application of Intrepide™ – preclinical animal test data
Animal trials were performed at the American Cardiovascular Research Institute (ACRI) (Atlanta, GA, USA) with GLP compliance. Documentation of all implant procedures and devices used, are kept on file at ACRI. Each animal underwent two invasive procedures. During the first, study stents to be implanted were divided into the following groups (bare stents, stents with the Parylene barrier coating alone, and stents coated with 60, 350, 500 or 750 µg Trapidil and a 3.0 mm Parylene barrier). Each group consisted of five stents that were randomised during implantation. Stents were implanted into the coronary arteries of juvenile farm pigs. Under general anaesthesia and using sterile surgical technique, the right femoral artery access was used and an 8 Fr sheath introduced. Heparin was administered intravenously to achieve an adequate activated clotting time (ACT). Selective coronary angiography was performed using standard angioplasty guide catheters, following administration of intracoronary nitrates. Coronary artery calibre was estimated and suitable sites chosen within the proximal segments to allow implantation of a 3.0 mm stent, oversized by approximately 15–20% to the luminal diameter. Each animal received two test stents in different coronary arteries. Stent deployment was performed under fluoroscopic guidance over a standard 0.014 inch angioplasty guidewire. Repeat angiography was performed following stent implantation to confirm adequate stent expansion and vessel patency. The femoral arteriotomy site was repaired and the leg wound closed. All animals were maintained on a standard laboratory chow diet throughout the study period. No animals were lost prior to completion.
At 28 days post-stenting procedure, animals had follow-up angiography to assess the degree of luminal narrowing in the two stented segments. The animal was then sacrificed. The heart was removed and perfusion fixed at 100 mmHg for 24 hours with 4% neutral buffered formalin.
Arteries containing stents were explanted and arterial sections containing the stents were sectioned and stained with EVG to visualise elastin. Additional arterial sections within 5.0 mm proximal and distal end of the stent as well as distal myocardium were sectioned and stained with Haematoxylin and eosin as well as EVG to determine any adverse events. Digital images were taken of the cross sectional area for each artery and stent section, and morphometry performed.
To determine if any delamination of barrier occurred in vivo, H and E and Masson’s Trichrome stains are used with sections derived from the same samples as the EVG stains. Digital images are taken at 10X and 20X objective magnifications and the area surrounding the stent struts observed. Average neointimal area was measure from stented segment for each group and is shown in Figure 4.
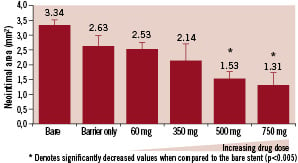
Figure 4. Average neointimal area.
The dose ranging visual assessment of the stents for neointimal hyperplasia is shown in Figure 5.
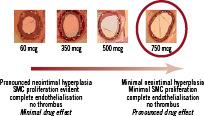
Figure 5. Dose ranging study in animal model before Destiny study.
It shows complete endothelialisation in each group with minimal neointimal hyperplasia and SMC proliferation in 750 µg dose.
Angiographic data was obtained from five replicate stents and analysed at ACRI. The collective results of this data are presented in Figure 6.
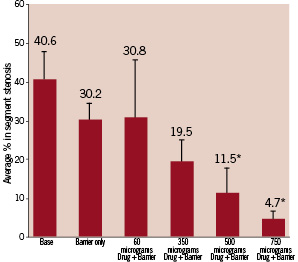
Figure 6. The collective angiographic data from five replicate stents.
While the average in segment restenosis reached 40.6% in bare stent controls, 500 and 750 µg of Trapidil loading levels significantly decreased to 11.5% and 4.7% respectively. There is thus an excellent dose response relationship.
Although the Parylene only controls displayed a trend towards a decrease in restenosis rates, no significance was established when compared to the bare stent controls.
Clinical experience
First in Man Study (DESTINY)
This was a prospective, multicentre study involving 100 patients (two were excluded after recruitment, but included as intention-to-treat) with de novo coronary lesions requiring single drug eluting stents of no longer than 16 mm length from 2.5 mm to 4 mm in diameter. The primary objective of the study was to show significant improvement in in-stent late loss for the Trapidil eluting stent compared to historical bare metal stent (BMS) controls. The primary hypothesis was to show late loss within the stent to be < 0.5 mm. The study achieved the primary endpoint but not the primary hypothesis.
This study demonstrated good deliverability of the stent, achieving 98% device success and 100% procedural success. Furthermore safety of the device was demonstrated as well, with freedom from MACE (Death, MI, CABG, TLR, TVR, TVF and SAT) at 30 days. At 180 days follow-up angiographic late lumen loss was 0.72±0.49, which was above the proposed hypothesis but significantly lower than the historical control. The percentage stenosis was 36.5 and binary stenosis was 9.5%. The TLR was 6.2%. There was no MI at 180 days and no subacute thrombosis (SAT) or late stent thrombosis (LST) demonstrating safety of the device. One death occurred in a patient (due to renal failure), following revascularisation surgery.
The study thus demonstrated that the Intrepide™ DES (Trapidil eluting) is a very deliverable, safe and effective device; however primary hypothesis of 50% reduction in late loss was not met. Further refinement in the manufacturing process has improved the elution profile. Analysis of the drug delivery data in animal models indicated that stents with appropriate drug load had definite improvement in late lumen loss. Another interesting observation of the study was low TLR despite relatively higher late lumen loss. The TLR noted here is very similar to those achieved in larger trials with Paclitaxel and Sirolimus eluting stents
PATIENT APT (PAtients Treated with the INTREPIDE™ Trapidil Eluting steNT on a reduced Anti- Platelet Therapy regime): Multicentre prospective registry
This open multicentre prospective registry trial was designed to assess the clinical event rate following treatment with Intrepide™ Trapidil eluting coronary stent(s) during PCI with a reduced duration of dual antiplatelet regime in patients with symptomatic coronary artery disease in one or more coronary arteries. Inclusion criteria were broad with few exceptions. Intervention of unprotected left main stem, Vein graft disease and in-stent restenotic disease were excluded.41,42
Primary objectives included clinical success during intervention, at discharge and follow-up of 30 days, six months and 12 months. Clinical success was defined as procedural success without the occurrence of major adverse cardiac events (MACE) of death, myocardial infarction (MI) and target vessel revascularisation (TVR) with re-PCI or CABG.
The secondary objectives included procedural and device success. Procedural success was defined as a 20% or less residual stenosis post angioplasty and stenting of all angiographic views with regard to the treated stenosis, on visual assessment, or quantitative angiography and TIMI III flow before the guiding catheter is removed. Device success is defined as successful delivery of the device (Intrepide™ stent) at the target lesion and achieving 20% or less residual stenosis post stenting in all views and TIMI III flow at the end of the procedure.
A total of 105 ‘real world’ patients requiring coronary intervention of one or more de novo coronary arteries were entered prospectively at multiple centres. They were treated with one or more 2.5 to 4.0 mm wide and 8 to 32 mm long TES. Only aspirin was continued instead of DAPT after three months (78 patients) at physician’s discretion. The mean age was 54±10 (range 30-84 years). Eighty three percent were men, 49% were diabetic (92% of them type II) and 69% lesions were complex (B2-C). Procedural and device success was 100%. Twelve patients had double vessel treatment. Mean stent diameter was 2.74 (±6). Mean length of stented segment was 21.8 (8-48 mm).Sixty seven percent patients required 20 mm or longer stents. Mean stented length for lesion was 22.2 (8-48) and 21.2 (8-40) in diabetics and non-diabetics respectively (p<0.1 ns). Mean follow-up was 11.7 months (range 6-19). Five patients required re-intervention (three diabetics). MACE rate was 5.7% with no mortality or LST. TES appears safe and effective with low rate of TLR and MACE in this ‘real world’ patient population with equal effectiveness in diabetics and non-diabetics despite shortened DAPT. Subgroup analysis of the diabetic population and patients with long lesions has shown excellent clinical performance regardless of the adverse risk factors. In terms of ‘real world’ statistics, there are several trials with established DES systems to whom the Intrepide™ TES could measure up in terms of clinical outcome.
Future clinical studies
Despite reducing the incidence of restenosis, the advent of DES has highlighted the need of greater vigilance as regards their long term safety especially in ‘real world’ patient populations. Most pivotal trials for regulatory approval have recruited patients of relatively low risk, the so called ‘on label’ group, however in practice DES is quite commonly used in complex lesion and clinical situations. A greater focus on safety of DES is therefore warranted. Large-scale observational surveillance studies with robust follow-up are required to establish the long-term safety of DES in real world populations. It should be mandatory and regulatory obligation for all manufacturers of recently approved / CE Certified DES to obtain and maintain a proper and reliable surveillance registry of these devices. It is planned that large scale multicentre post-market surveillance studies will be carried out of Intrepide™ stent for ‘all comers’ in Europe and rest of the world to be called I-CLARITY EUROPE and I-CLARITY ROW respectively. These registries will ensure a robust follow-up and data collection of all patients following Intrepide™ stent implantation. It will focus on deliverability, efficacy and safety of the device for ‘real world’ patients on medium and long-term basis.
Conclusions
Trapidil DES is an interesting device coated with a drug which has wide ranging modes of action. Trapidil being a non-cytotxic drug and not utilising the mTOR pathway, the risk of delayed healing is significantly reduced thereby reducing the risk of LST.43 The unique actions of Trapidil may also reduce the need for long-term /indefinite dual antiplatelet therapy.
The cascade of preventing the neointimal hyperplasia here does not involve a cytostatic or cytotoxic pathway. The mode of action of Trapidil is such that it prevents neo-intimal hyperplasia, yet may not unduly hamper endothelial healing following percutaneous coronary intervention. It demonstrated its effectiveness in real-world complex lesion subset with equal efficacy in diabetics and non-diabetics. Trapidil eluting devices, therefore, offer great potential in the prevention of restenosis, especially for those who are unable to take long term dual anti-platelet therapy (DAPT). The thin struts help in the placement of stents with a very high rate of success. The safety of the device was well demonstrated in both above mentioned studies with no MACE at 30 days and no late stent thrombosis (LST) at 180 days. It is thus a safe and effective device with excellent medium term outcome.

