Abstract
Background: As non-surgical percutaneous interventions are increasingly considered for many cardiac conditions, high quality near field continuous imaging is warranted, in order to optimize the results, and to prevent and detect complications. Transesophageal echocardiography is the standard imaging technique, however general anesthesia and endotracheal intubation is required during prolonged monitoring of percutaneous interventions. Intracardiac echocardiography is a novel emerging tool that allows a view within the cardiac chambers and the large vessels and can be employed by the interventional cardiologist.
Method: In our department, a phased array, multi-frequency, four-way steerable catheter (AcuNaV™ - Siemens) was used for anatomic and haemodynamic cardiac assessment and for guidance and monitoring during non-coronary percutaneous interventions. In total 135 patients underwent intracardiac echocardiographic investigation, 4 during diagnostic heart catheterization, 6 during percutaneous coronary intervention with the use of a new left ventricular assist device, the Impella Recover LP 2.5 system, 26 during percutaneous transluminal septal myocardial ablation (10 patients were reevaluated with intracardiac echocardiography at 6 months), 50 during interatrial communication closure, 4 during percutaneous left atrial appendage transcatheter occlusion, 7 during percutaneous balloon valvuloplasty, 1 during percutaneous aortic valve replacement and 27 during pulmonary vein ablation. All patients tolerated the procedure very well with no catheter related complications. However, there were two complications, which were due to the guidewire and the sheath, an inferior vena cava dissection and a femoral vein dissection, respectively.
Conclusion: Phased array intracardiac imaging is a safe technology, which facilitates non-surgical interventions by providing high quality images. It eliminates the need for general anesthesia and thus increases the patient comfort.
Introduction
Percutaneous interventions have been widely accepted as an alternative to surgical treatment for various congenital and acquired cardiac conditions. Near field high quality images are a prerequisite for optimization of results, as well as for detection and avoidance of complications during procedures, such as interatrial communication closure, percutaneous transluminal septal myocardial ablation (PTSMA), percutaneous left atrial appendage closure (PLAATO), and pulmonary vein ablation. High quality images guide transseptal catheterization, whereas delineation of the endocardium facilitates mapping and radiofrequency ablation by identification of target area, verification of tissue electrode contact, and titration of energy.
Conventional fluoroscopy is restricted by its inability to discriminate soft tissue, and its intrinsic limitation to provide only two-dimensional cross-sections. Thus, although it allows visualization of the devices inside the cardiac silhouette, accurate positioning of the catheters may be difficult. On the other hand, transthoracic echocardiography cannot show the posteriorly situated, remote areas, especially in the supine position. Transesophageal echocardiography is the standard imaging modality in the catheterization laboratory during interventions for monitoring as well as for guiding the procedure. However, in a supine position, general anesthesia and endotracheal intubation to protect the airways are frequently required. This may increase procedure time and cost and may add to the complications, even though the incidence of such complications is rare.
The first attempts to percutaneously introduce in vivo intravenous probes with built in echo transducers for intracardiac imaging were reported in the late 60s1,2. During the following years, several intracardiac echocardiography (ICEC) catheters were developed3-9. Human clinical studies demonstrated that ICEC is a feasible and safe modality, which allows recording of structures that may be difficult or impossible to obtain with routine external echocardiography3-5. Further miniaturization of ICEC technology has resulted in smaller imaging probes that can be easily manipulated, in order to optimally visualize the cardiac anatomy from within the right cardiac chambers. Moreover, it avoids the need for general anesthesia, increases the patient comfort and tolerance and can be performed by the interventional cardiologist (Table 1).
Currently, two type of ICEC catheters are commercially available and used in clinical practice: a) mechanical-single element rotating system and b) phased array multiple element transducers. They both provide comprehensive anatomical information in real time, whereas the second also has full Doppler capability incorporated. We report our initial experience with the AcuNaV™ intracardiac probe in various interventional procedures.
Method
Study population
We investigated with ICEC a total of 135 patients (pts) scheduled for diagnostic heart catheterization or percutaneous cardiac intervention. More specifically, 4 pts were evaluated during diagnostic heart catheterization, 6 pts during percutaneous coronary intervention with the use of a new left ventricular assist device, the Impella Recover LP 2.5 system, 26 pts during percutaneous transluminal septal myocardial ablation (PTSMA) (10 pts were reevaluated with ICEC at 6 months), 50 pts during interatrial communication closure, 4 pts during percutaneous left atrial appendage transcatheter occlusion (PLAATO), 7 pts during percutaneous balloon valvuloplasty, 1 patient during percutaneous aortic valve replacement and 27 pts during pulmonary vein ablation (PVA) or isolation. In table 2 are shown the various types of intervention performed under ICEC guidance.
Intracardiac echocardiography
EQUIPMENT DESCRIPTION
The AcuNaV™ - intracardiac probe (Siemens) is a 10F catheter equipped with a linear phased array multi-frequency (5.5 to 10 MHz) transducer (Figure 1).
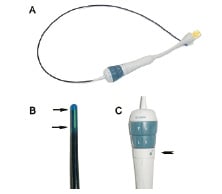
Figure 1. The AcuNaV™ - intracardiac probe (A) is a 10F catheter equipped with (B) a linear phased array multi-frequency transducer (arrows). (C) External manipulation of the proximal part (arrows) permits four way steering in two planes, anterior-posterior and left-right. The tension control knob (arrowhead) secures the catheter in the desired position.
It offers 90° sector imaging, has a changeable ultra sound frequency (5.5, 7.5, 8.5, 10 MHz) depending on the applied console, and a penetration capacity of 15 cm. External manipulation of the proximal part permits four way steering in two planes, anterior-posterior and left-right with 160º rotation, enabling complete view of the heart from the right chambers. The tension control knob secures the catheter in the desired position. Color and pulse Doppler provides information on blood flow and velocity. The insertable part of the catheter is 90 cm in length. Measurements and calculations can be done online or offline and include left ventricular volumes, function and haemodynamics. Images may be acquired by interfacing with a standard ultrasound system (Cypress™ or Sequoia™, Siemens) and can be digitally recorded thereafter in three different formats (DICOM, AVI and the console format).
INTRACARDIAC ECHOCARDIOGRAPHY IMAGING
The probe is inserted in the right or left femoral vein through a 12F long sheath, which is introduced over a stiff wire (Mayer wire™, Boston Scientific). Under fluoroscopic guidance in the anteroposterior view the catheter is advanced through the inferior vena cava into the mid right atrium, without the application of steering or tension. In this position, the long axis of the right ventricle, the tricuspid valve and part of the right atrium is imaged (Figure 2).
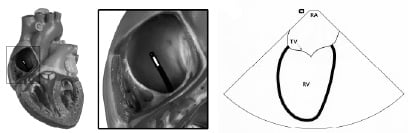
Figure 2. (A) The catheter is advanced through the inferior vena cava into the mid right atrium. (B) In this position, the long axis of the right ventricle (RV), the tricuspid valve (TV) and part of the right atrium (RA) is imaged.
Clockwise rotation provides views of the outflow tract of the right ventricle, the pulmonary artery, the aorta, the tricuspid valve and the right atrium (Figure 3).
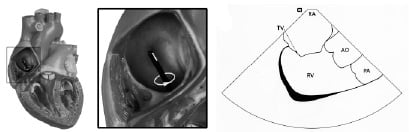
Figure 3. (A) Clockwise rotation of the catheter at the mid atrium provides views (B) of the outflow tract of the right ventricle (RV), the pulmonary artery (PA), the aorta (AO), the tricuspid valve (TV) and the right atrium (RA).
The left atrial appendage and the mitral valve are delineated with additional clockwise rotation and possible left steering. Further advancement of the catheter or posterior steering of the tip enables visualization of the entire atrial septum and the fossa ovalis (Figure 4).
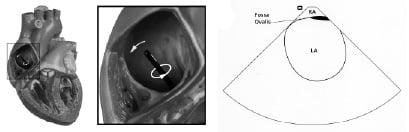
Figure 4. (A) Further advancement of the catheter or posterior steering of the tip enables visualization of (B) the entire atrial septum and the fossa ovalis.
Supplementary right or left steering may be required for optimal visualization of the fossa ovalis. From the interseptum view, the left pulmonary veins can be imaged by advancing the probe without applying any tension or steering. Rotation of the catheter clockwise and insertion in to the superior vena cava allows the right pulmonary veins to be visualized in a cross-sectional view. Left or right steering enables long-axis view of the right pulmonary veins. In order to enter the right ventricle, the tip of the probe is positioned in the mid to upper right atrium with the piezo-electric crystal facing the free wall of right atrium and then is gradually deflected anteriorly. Thereafter, the long axis of the left ventricle is obtained, where proximal interventricular septum (IVS), left ventricular outflow tract (LVOT) and mitral valve apparatus are visualized (Figure 5).
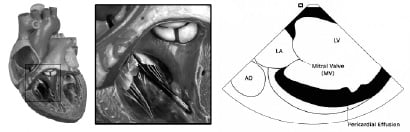
Figure 5. (A) The catheter is advanced through the tricuspid valve and is rotated clockwise in order to visualize the left ventricle. (B) Long axis of the left ventricle. MV: Mitral Valve, AO: Aorta, LV: Left Ventricle, LA: Left Atrium.
Procedures
ANATOMIC AND HAEMODYNAMIC CARDIAC ASSESSMENT
We applied ICEC in 4 pts undergoing diagnostic heart catheterization and in 6 pts during percutaneous coronary intervention with the use of a new left ventricular assist device, the Impella Recover LP 2.5 system. In one patient with aortic stenosis we assessed the configuration and the extent of calcification of the annulus and the cusps, the degree of left ventricular hypertrophy and the functional status of the other valves. Additionally, we evaluated the potential presence of aortic insufficiency, obstruction of the left ventricular outflow tract or aortic dilatation.
The cause of mitral regurgitation was investigated in one patient with acute inferoposterior myocardial infarction and cardiogenic shock during primary angioplasty, as there was no haemodynamic improvement following revascularization and intraaortic balloon support. A long axis view from the right ventricle enabled evaluation of the mitral valve and leaflets (Figure 6).
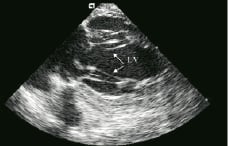
Figure 6. Long axis view of the left ventricle (LV) showing the intact mitral apparatus (arrows) with the probe positioned into the right ventricle.
ICEC confirmed that the mitral apparatus was intact, whereas severe hypokinesia of the inferoposterior wall was observed.
One patient was studied with ICEC because of tricuspid valve prosthesis (St. Jude valve) dysfunction, which was difficult to be visualized with transthoracic or transesophageal echocardiography. ICEC revealed severe prosthetic valve stenosis and moderate regurgitation, due to the presence of a large thrombus.
An unusual case of a patient with multiple congenital abnormalities (bicuspid aortic valve, left cervical aortic arch, two aortic coarctations) and two aortic valve replacement operations in the past was investigated with ICEC. In the particular patient, aortic gradient measurements were obtained with the insertion of a Swan-Ganz catheter in the left ventricle. Interatrial septum puncture was performed under ICEC guidance. Furthermore, ICEC imaging enabled visualization of the coarctation in the ascending aorta and colour Doppler interrogation revealed turbulent flow10.
Finally, left ventricular diastolic and systolic areas were calculated during the use of the Impella Recover LP 2.5 system in 6 pts undergoing percutaneous coronary intervention.
PERCUTANEOUS TRANSLUMINAL SEPTAL MYOCARDIAL ABLATION IN HYPERTROPHIC OBSTRUCTIVE CARDIOMYOPATHY
We evaluated the application of ICEC for the identification and measurement of the area at risk for alcohol necrosis, after intracoronary contrast injection in 26 symptomatic pts who underwent PTSMA (Table 3).
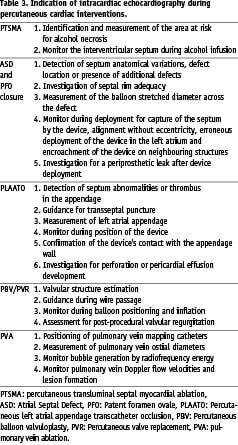
M-mode of the mitral valve confirmed systolic anterior motion (SAM) and thickness of the IVS (Figure 7).
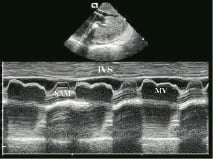
Figure 7. Intracardiac M-mode in a patient with hypertrophic obstructive cardiomyopathy demonstrating systolic anterior motion (SAM) of the mitral valve (MV). Interventricular septum: IVS
The left ventricular outflow tract and mitral valve were interrogated with Doppler, so as to detect flow acceleration and mitral regurgitation. The entire proximal and mid septum were displayed for further imaging during myocardial contrast injection and ablation. Coronary angiography was performed in multiple projections to delineate the septal perforator. One ml of myocardial echo contrast (SonoVue™, Bracco International B.V., Netherlands) was injected into the proposed septal branch during balloon occlusion. Accordingly, the area at risk for alcohol necrosis was identified and measured by planimetry (Figure 8A).
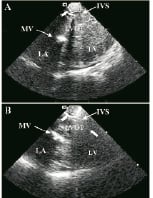
Figure 8. (A) Interventricular septal (IVS) imaging after 1 ml of myocardial echo-contrast injection (arrowhead). (B) During alcohol administration progressive appearance of back scattering (arrowhead) is seen. Highly echogenic area with shadowing into left ventricular outflow tract (LVOT) at the end of the ablation (arrow) Mitral Valve: MV, Left Ventricle: LV, Left atrium: LA.
Subsequently, PTSMA was performed according to the standard protocol11. Images obtained during alcohol infusion revealed progressive appearance of a sharply demarcated triangular area with increased echo density in the septum (Figure 8B) producing a marked shadowing effect. This echo-dense area was larger than the area delineated by myocardial contrast injection. Alterations of myocardial echo contrast optical properties during exposure to alcohol in addition to the presence of alcohol in the myocardium due to capillary leak may be the reason for the development of this highly echogenic and larger area during ablation. Recruitment of additional septal perforators was not required in these patients, as left ventricular outflow tract gradient decline was satisfactory after treatment of the septal branch initially selected. The procedure was completed after obtaining long axis 2D and M-mode images. All pts had satisfactory reduction in left ventricular outflow tract gradient. There was only one sheath related complication, a femoral vein dissection, which was conservatively treated with good result. In 10 pts follow-up angiography and ICEC was performed at six months.
TRANSCATHETER CLOSURE OF INTERATRIAL COMMUNICATIONS
We assessed the use of an ICEC probe in guiding closure of atrial septal defects (ASD) and patent foramen ovale (PFO) as an adjuvant to fluoroscopy in 50 consecutive pts (Table 3). ICEC probe was introduced into the right atrium as described earlier. The defect size and location, adequacy of the septal rims, and the adjacent structures were assessed in all pts with ASD (Figure 9).
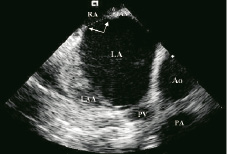
Figure 9. Transverse axis of the interatrial septum (IAS) showing the secundum atrial septal defect (ASD) and rims (arrows). Right atrium: RA, Left atrium: LA, Left atrial appendage: LAA, Aorta: Ao, Pulmonary Vein: PV, Pulmonary Artery: PA.
After color Doppler application (Figure 10) the septum was further scanned for potential additional defects.
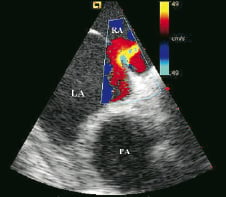
Figure 10. Colour doppler of the atrial septal defect (ASD) demonstrating left to right shunt. Right atrium: RA, Left atrium: LA, Pulmonary Artery: PA.
In pts with PFO (Figure 11), the interatrial shunt was confirmed by manifestation of contrast in left atrium with or without valsalva maneuver (Figure 12).
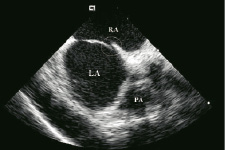
Figure 11. Patent foramen ovale (PFO) without septal aneurysm (cycle). Right atrium: RA, Left atrium: LA, Pulmonary Artery: PA.
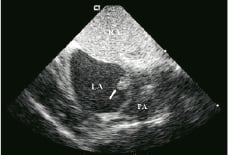
Figure 12. A patent foramen ovale (PFO) documented by micro bubbles entering the left atrium after contrast injection (arrow). Right atrium: RA, Left atrium: LA, Pulmonary Artery: PA.
Septal aneurysms were present in more than 40% cases of PFO, but they did not interfere with visualization of the rest of the septum and device closure in this small cohort of pts. The septum was redundant in two patients. Wire placement across the PFO was facilitated by ICEC (Figure 13) and in 1 patient additional fenestration was diagnosed while probing.
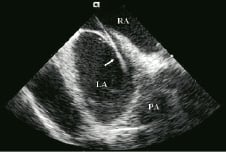
Figure 13. A wire across the foramen ovale (PFO) (arrow). Right atrium: RA, Left atrium: LA, Pulmonary Artery: PA.
Thereafter, the diameter of the stretched balloon across the defect was measured both by echocardiography (Figure 14) and fluoroscopy.
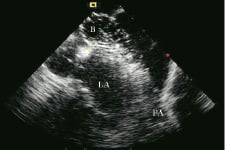
Figure 14. Stretched diameter by intracardiac echo during balloon (B) sizing of atrial septal defect (ASD). Left atrium: LA, Pulmonary Artery: PA.
We selected the device according to balloon sizing. Closure was performed according to the standard protocol12. During device deployment, alignment of the device, relation to neighboring structures especially atrioventricular valves and capture of the septum by the device were closely observed in orthogonal planes (Table 3). The procedure was completed with colour Doppler interrogation for periprosthetic leak in ASD, in order to verify absence of contrast in the left atrium under a valsalva maneuver and to confirm the complete closure of PFO. Successful closure (Figures 15 and 16) was achieved in all pts and there were no complications related to either ICEC or device.
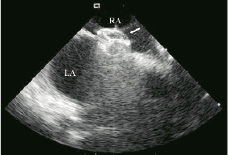
Figure 15. Appropriately deployed Amplatzer device (arrow) for atrial septal defect (ASD) occlusion in short axis. Right atrium: RA, Left atrium: LA.
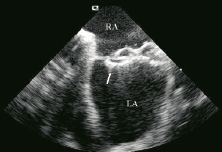
Figure 16. Completely deployed Cardioseal Starflex device (arrow). Right atrium: RA, Left atrium: LA.
PERCUTANEOUS LEFT ATRIAL APPENDAGE TRANSCATHETER OCCLUSION
We performed 4 PLAATO procedures under intracardiac echo monitoring (Table 3). Transeptal puncture was performed without any complication under ICEC guidance after excluding abnormalities of the septum. In all pts tenting of the fossa ovalis by the transseptal needle was readily detected (Figure 17).
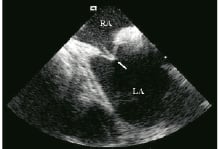
Figure 17. Transseptal puncture under intra cardiac echo (arrow). Right atrium: RA, Left atrium: LA.
The left atrial appendage ostium could be easily delineated and measured by ICEC. Accordingly, positioning, release, periprosthetic leak and relation of the device to surrounding structure were monitored by ICEC. Intracardiac imaging enabled good visualisation and confirmation of the device’s contact with the inner wall of the left atrial appendage.
PERCUTANEOUS BALLOON VALVULOPLASTY OR VALVE REPLACEMENT
We performed percutaneous aortic valve replacement in a very sick patient, who was not amenable to undergo surgery, due to concomitant severe non-cardiac disease. The aortic annulus was well delineated by ICEC. Additionally, ICEC facilitated crossing the valve with the wire by providing a transverse axis view of the valve with the central orifice. Percutaneous balloon valvuloplasty under ICEC imaging was performed in 3 pts with mitral valve stenosis, in 3 pts with aortic stenosis (Figure 18) and in one 18 weeks pregnant woman with triple-valve stenosis (mitral, tricuspid and aortic valve) due to rheumatic heart disease.
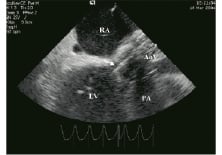
Figure 18. Percutaneous aortic valvuloplasty in a patient with severe aortic stenosis. Intracardiac echocardiography facilitates the insertion of the wire and the balloon (arrow) through the heavily calcified and stenotic AoV. Left ventricle: LV, Pulmonary artery: PA.
In all cases ICEC provided excellent imaging and guidance, which resulted in a low radiation exposure and minimised the need for contrast angiography. Inferior vena cava dissection caused by the guidewire during the sheath introduction was observed in one patient who underwent aortic valvuloplasty. After the procedure the patient was admitted in the coronary care unite for observation and there was no need for surgical repair.
PULMONARY VEIN ABLATION
Twenty-seven patients underwent PVA for treatment of atrial fibrillation (Table 3). Double transseptal puncture for catheterization of the left atrium was directed by ICEC. A multipolar basket catheter and thereafter an ablation catheter were positioned into the target pulmonary vein. Segmental isolation of the pulmonary veins was performed at the veno-atrial junction, guided by the local electrograms. Doppler measurements of transvenous flow velocity were performed before and after ablation. During the procedure the ablation zone was directly visualized, permitting assessment of shape, cross-sectional area, wall thickness and lesion formation. Additionally, ICEC was used to continuously monitor bubble creation throughout radiofrequency energy delivery, in order to prevent overheating of the tip of the catheter. After ablation, flow velocities were interrogated in order to identify the potential presence of a gradient. In this study group, no patient developed acute severe pulmonary vein stenosis and no embolic events were detected.
Discussion
Evaluation of ventricular and valvular function is indispensable in pts with either coronary or valvular heart disease. Thus, in such pts it is not only necessary to gain anatomical and haemodynamic information, but also to estimate the functional status of the left ventricle and the valvular apparatus. In the clinical setting, ICEC has been proven useful for direct measurement of the aortic valve area with an accuracy similar to the invasive and noninvasive methods currently used13. We used AcuNav™ intracardiac probe in various coronary and valvular heart disease procedures. The device provides standard echocardiographic views hence orientation is easy. We had no device related problems and all pts tolerated the procedure well. There was only one sheath and one guidewire related complication.
PTSMA has emerged as an alternative to myectomy for hypertrophic obstructive cardiomyopathy pts with drug-refractory symptoms14,15. The proper selection of the septal branch perfusing the septal segment involved in the obstruction is essential to obtain the therapeutic result and to reduce complications. Different methods have been used over time to identify the target vessel16. Assessment of gradient reduction after probationary balloon inflation has been substituted by visualisation of the risk area with myocardial contrast echocardiography under transthoracic imaging. We used ICEC to identify the area at risk for alcohol necrosis, to confirm the septal myocardium responsible for gradient genesis and to rule out aberrant supply of the papillary muscles and free ventricular wall by the selected septal branch.
ASD and PFO closure procedures are increasingly performed, as a treatment option in otherwise normal individuals with recurrent transient ischemic attacks or strokes (cryptogenic stroke)17-19. Balloon sizing of the defect with fluoroscopy or transesophageal echocardiography is standard practice12. We applied ICEC in order to clearly document, prior to the procedure, septum anatomical variations, defect location or additional defects, and septal rim adequacy. Furthermore, during the intervention, deployment process was continuously monitored, in order to avoid complications such as capture of the septum by the device, malalignment with eccentricity, erroneous deployment of the device in the left atrium, encroachment of the device on neighbouring structures especially the aortic valve, pericardial effusion and thrombus formation. A small residual leak is not uncommon, even after closure with an exact size device and it usually disappears in the course of time. However, after deployment in our pts no periprosthetic leak was observed. Hijazi et al. compared ICEC and transesophageal echocardiography employment for guiding atrial defect closure in 11 patients with either secundum ASD or PFO20. They demonstrated that both techniques provide similar views of the defects and the device deployment, whereas the images obtained by ICEC were more helpful and informative than those obtained by transesophageal echocardiography. Furthermore, Bartel et al. showed that ICEC guided closure of atrial septal defects results in reduced fluoroscopy and procedural time21. Recently, Zanchetta et al. reported their 3-year follow-up experience of transcatheter atrial septal defect closure assisted solely by ICEC22.
Atrial fibrillation is a common cause of embolic stroke and the majority of thrombi derive form within the left atrial appendage23. There are three fundamental approaches to the management of atrial fibrillation: rate control, rhythm control, and anticoagulation24,25. PLAATO is proposed as an option in those with a contraindication to oral anticoagulation26. Anatomical variation of the appendage, presence of thrombus and the diameter of the ostium are usually assessed by TEE followed by intra procedural monitoring. In addition to the conventional closure technique one has to establish access to the appendage by atrial septal puncture. Johnson et al. in the experimental setting investigated the application of ICEC for transseptal catheterization27. Multifrequency imaging of the membranous fossa ovalis, posterior left atrium, and left atrial appendage was performed from the right atrium. In all cases, membranous fossa ovalis «tenting» identified contact of the needle with the interatrial septum, while transseptal crossing and advancement of the dilator and sheath were adequately imaged27. We performed PLAATO in 4 pts, under ICEC guidance for transseptal access to the left atrium. Complete apposition of the device in the left atrial appendage was also assured for the prevention of device embolization or potential thrombus formation. In addition, ICEC allowed close monitoring for possible procedural related complications such as perforation and pericardial effusion development.
Percutaneous balloon valvuloplasty or valve replacement is an alternative to surgical valve replacement. Intra procedure intracardiac echo may be considered as an optional imaging modality, when the transthoracic acoustic window is very poor, for valvular structure estimation and when enhanced views are required. Salem et al. reported successful percutaneous balloon mitral valvuloplasty done under ICEC guidance28. Huber et al. implanted into the aortic valve of 6 calves an equine pericardial valve mounted onto a self-expanding nitinol stent29. During the procedure, leaflet motion, transvalvular gradient, regurgitation and paravalvular leaking were evaluated with ICEC and intravascular ultrasound29.
Interventional electrophysiology is rapidly evolving, and the demand for imaging guidance is more necessary than ever. Currently available modalities, such as fluoroscopy and endocardial ECG recordings to guide interventions, while helpful, have certain limitations. The use of ICEC may provide additional information, enabling accurate anatomical positioning of the ablation catheter tip in relation to adjacent endocardial structures30,31, visualizing evolving tissue injury, confirming lesion formation32,33, and detecting lesion size and continuity34. Moreover, ICEC has been shown to contribute to the safety of the procedures and to the prevention of potential complications associated with certain electrophysiological interventions35,36. All of these benefits certainly apply to the pulmonary vein ablation for the treatment of atrial fibrillation. This treatment modality is being used widely in ever increasing numbers, particularly in pts resistant to therapy37-39. The strategy of following the ablation by monitoring bubble formation improves efficacy and results in less early and late complications40.
Recent advances in ICEC, such as 3D imaging may significantly improve visualization of cardiac anatomy. Three-dimension ultrasound systems consist of a device that automatically pulls back the catheter stepwise, gated by the R-R and respiration signal of the patient. Post-processing algorithms transform two-dimensional image slices into a 3D representation of the anatomical structures (TOMTEC™ imaging systems, Unterschleissheim, Germany)41. Preliminary studies have demonstrated that, three-dimensional echocardiography has opened new perspectives for more effective and accurate imaging during electrophysiology procedures41-43.
Limitations
ICEC has been successfully applied in various procedures. Although, simple to use, it requires a learning curve and an adequate experience with transthoracic and transoesophageal echocardiography, even for an interventionalist familiar with right heart catheterisation. Linear phased array intracardiac catheters are an emerging promising diagnostic modality, but they have limited sector imaging compared to probes used for transesophageal echocardiography. Furthermore, because of the close proximity of the transducer and the interventricular septum, no exact septal measurements are possible. Imaging the heart from within the right chamber appears feasible and eliminates the need for general anaesthesia, and intubation, thus increasing the patient’s comfort. On the other hand, it adds to the cost of the procedure. Additionally, the multi-frequency probe does not provide harmonics.
Conclusion
Non-surgical cardiac procedures require real time, high quality and near field views for optimisation of results. Moreover, continuous progress in the field of percutaneous interventions warrants more effective imaging guidance, without compromising patients’ comfort and safety. ICEC is capable of identifying distinct endocardial structures with excellent resolution and detail. Furthermore, ICEC has been proven an important diagnostic tool for the detection of known or unanticipated aberrant anatomy in patients with congenital heart disease. Tissue motion, intracardiac devices and their relation to the surrounding structures can be very clearly delineated. Additionally, ICEC can provide a complete Doppler examination of intracardiac and paracardiac flows, allowing comprehensive haemodynamic assessment. In the future, ICEC may find a place in operating rooms and catheterisation laboratories for online monitoring of complex intracardiac interventions. Nevertheless, its incremental value in guiding and optimising such procedures warrants further evaluation in a larger number of pts.
Acknowledgments
The authors would like to express their gratitude to Paula Delfos and Jan Tuin for the photographs of the Acunav catheter positioning in the heart chambers.

