Abstract
Objectives: The TAXUS® database was used to perform an integrated analysis across several large phase II and III trials to assess outcomes of polymer-based paclitaxel-eluting TAXUS stents in diabetic patients.
Background: While drug-eluting stents have reduced the risk for restenosis, diabetic patients remain at higher risk.
Methods: This post hoc analysis of clinical and angiographic outcomes combined individual patient data from four randomized, multicentre, controlled clinical trials (TAXUS II, IV, V, and VI) representing outcomes across two paclitaxel release formulations and stent platforms.
Results: Breslow-Day analysis indicated homogeneous odds ratios across dose formulations and stent platforms, thus clinical data (9 months) were pooled from 3445 patients, including 814 diabetic patients. The randomized quantitative coronary angiography subset (n=2863) included 469 diabetic patients requiring oral medications only and 216 insulin-treated. Among patients receiving bare metal stents (BMS), those with diabetes had worse clinical and angiographic outcomes than non-diabetic patients. Target lesion revascularization rates were decreased in TAXUS patients relative to BMS controls by 59% (p=0.0001) among diabetic patients requiring oral medications, by 66% (p=0.006) among insulin-treated. Insulin-treated patients showed a significant TAXUS benefit for in-stent and in-segment percent diameter stenosis (p=0.0001, p=0.001, respectively) and late loss (p=0.001); a similar TAXUS benefit was seen in diabetic patients treated with oral medications (p<0.0001). Binary restenosis in-segment was significantly decreased 65% in both diabetic subsets (p=0.0001).
Conclusion: This post hoc analysis of data from four combined randomized TAXUS trials suggests the TAXUS benefit observed in non-diabetic patients is carried over into the high-risk diabetic population.
Introduction
The prevalence of diabetes is increasing at an alarming rate world wide.1 As one important complication, diabetic patients have been shown to develop an accelerated form of coronary atherosclerosis associated with high morbidity and mortality. Diabetic patients are often resistant/refractory to therapeutic and interventional treatments for coronary artery disease, with high rates of disease progression and restenosis, including higher rates of restenosis following implantation of bare metal stents.2-8 It has been suggested that this resistance might be explained in part by restenotic mechanisms which may differ from those in the general population, reflecting the diffuse and aggressive nature of vascular disease in diabetic patients.9,10
Diabetic patients account for approximately 20-25% of patients undergoing percutaneous coronary intervention as confirmed in trials of drug-eluting stents (DES).3-8,11,12 The TAXUS program includes four large trials with increasing lesion complexity, as specified by the inclusion and exclusion criteria, that have reached their primary endpoint. In these trials, the percentage of medically treated diabetic patients defined by the medication profile has ranged from 18% to 32%.13-17 Assessment of the effectiveness in the diabetic subgroup has been limited by the small sample size in each of the individual randomized clinical trials. While a post hoc subgroup analysis of each individual phase II and III TAXUS trials has demonstrated significant benefits for quantitative angiographic indices, the benefit failed to reach statistical significance for binary clinical indices of restenosis.
The overall objective of this report was to perform an integrated analysis using the individual patient data from four combined randomized TAXUS trials to evaluate pooled outcomes for diabetic patients receiving the TAXUS stent relative to control bare metal stents (BMS).
Methods
TAXUS Studies
The four largest TAXUS trials (TAXUS II, TAXUS IV, TAXUS V, and TAXUS VI described in Table 1)13-17 that were randomized, blinded, and with active controls were included in the integrated analysis because endpoint protocols and processes were harmonized to allow for subsequent pooling. Specifically, the endpoint definitions were preserved across the trials as was the use of an independent Clinical Events Committee (CEC), 100% monitoring, and independent Core laboratories.
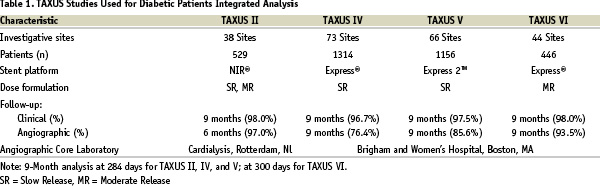
In these studies, the CEC has the ability to add additional events that may not have been reported by the investigative site. Three of the four studies (TAXUS IV, TAXUS V, and TAXUS VI) stratified for medically treated diabetes, thus facilitating balanced comparison of this high risk group. The program focused on capturing ‘medically treated diabetic patients’ because diabetic medications could be readily confirmed through monitoring the medical records which were available to the interventional cardiologists.
The primary endpoint of the study was the rate of target vessel revascularisation (TVR) nine months after the study procedure. Secondary endpoints included the rates of clinical procedural success, composite MACE [death, myocardial infarction or target lesion (TLR) and target vessel revascularization (TVR)] at 1, 3, 6 and 9 months after the study procedure and annually for 5 years, the Target Vessel Failure (TVF) rate and the stent thrombosis rate. Myocardial infarctions were categorized as Q-wave, and non-Q wave; Q-wave infarction was defined as the development of new pathological Q-waves in two or more leads lasting 0.4 seconds or more with post procedure CK-MB levels elevated above normal; non-Q wave infarction was defined as the presence of post-procedure CK levels >2.0 times normal with positive CK-MB.
Angiographic variables derived from the six month angiographic restudy in TAXUS II, and nine month restudy in TAXUS IV, V and VI included absolute lesion length, stent length, reference vessel diameter (RVD), minimum lumen diameter (MLD), percent diameter stenosis, binary restenosis rate, acute gain, late loss, loss index, and the patterns of recurrent restenosis, including the edge effect (i.e. measurements of the in-stent segment and the analysis segment, that is the in-stent segment plus 5 mm at either end).
Statistical analysis
Individual patient data were integrated from the four TAXUS trials (TAXUS II, TAXUS IV, TAXUS V, and TAXUS VI) into one common database representing outcomes across two paclitaxel release formulations [slow release (SR) versus moderate release (MR)] and two stent platforms (NIR® versus TAXUS Express2). For binary data, homogeneity of the odds ratios across the four TAXUS studies was assessed with the Breslow-Day test, which tests the null hypothesis that the odds ratios of the treatment effect across studies are equal.18 A p-value >0.05 in the test suggests there is insufficient statistical evidence of heterogeneity in the treatment effect, justifying pooling of the results. If the Breslow-Day test indicated a treatment-by-study interaction, then the quantitative interaction with treatment effect in the same direction but of different magnitude was investigated. If there was no evidence to contradict the assumption of homogeneous odds ratios across different TAXUS studies, the treatment effect of DES over BMS from the pooled data was assessed using a 2-sided Fisher’s exact test.18 Similarly, for continuous data, an analysis of variance (ANOVA) model was used to assess the treatment by study interaction and the treatment effect.
Results
Integrated analysis design
The key characteristics of the four TAXUS studies used in this integrated analysis are summarized in Table 1, which highlights the differences and similarities. Breslow-Day analysis to test for homogeneity indicated that the estimated treatment effects (odds ratios) across trials are homogeneous (all p-values >0.05, see Table 4 below), thus allow pooling of the data across stent platforms and formulations. Clinical data from 3,445 patients were pooled as shown in Figure 1, yielding 814 patients with medically treated diabetes compared with 2,631 non-diabetic patients as outlined in Table 2.

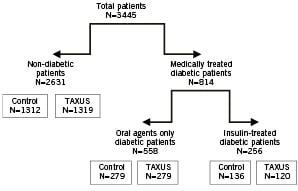
Figure 1. Integrated analysis design
Baseline characteristics
The baseline characteristics for diabetic and non-diabetic patients presented in Table 3 show that coronary artery disease in diabetic patients is found in smaller vessels (p<0.0001) and is characterized by more silent angina (p=0.005) and longer lesions (p=0.002).
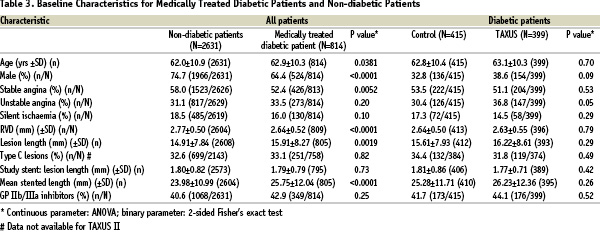
In the four TAXUS trials combined, there were proportionally more females among patients with diabetes than without (p<0.0001). The total stented length in diabetic patients was significantly longer (p<0.0001). Among medically treated diabetic patients the Control and TAXUS groups are evenly balanced for baseline characteristics; the one exception is a trend towards unstable angina in the TAXUS-treated diabetic patients (Table 3).
Pooled clinical results
Table 4 shows pooled clinical results at 9 months.
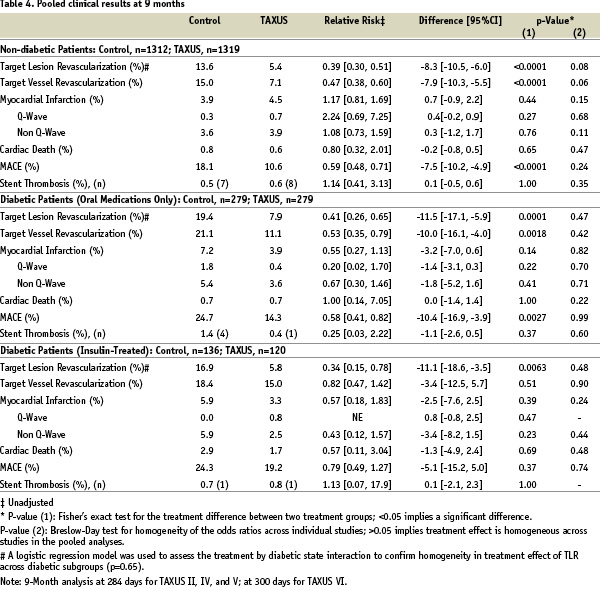
As expected TLR and TVR rates in non-diabetic patients receiving TAXUS versus BMS decreased by 47% (p<0.0001) and 60% (p<0.0001), respectively. Diabetic patients receiving BMS had worse clinical outcomes than did non-diabetic patients. However, patients taking oral medication who received the TAXUS stent had significant reductions in TVR compared to those receiving BMS (p=0.0018). Likewise, significant reductions in TLR were seen among diabetic patients receiving the TAXUS stent with 59% less (p=0.0001) among patients taking oral medications and 66% less (p=0.006) among those treated with insulin. Rates of cardiac death and myocardial infarction were similar between BMS and TAXUS. Hence, the composite major adverse cardiac event (MACE) rates, driven by TLR, were reduced by 41% (p<0.0001) in non-diabetic patients, to a similar extent (42%, p=0.003) in diabetic patients receiving oral medication, and by 21% in insulin-treated diabetic patients. This translates to significantly higher 9-month TLR-free survival rates for all three groups receiving the TAXUS stent (Figure 2).
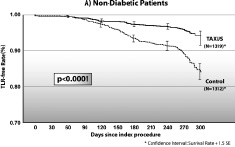
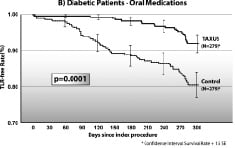
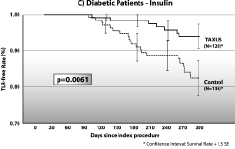
Figure 2. Survival curves for freedom from target lesion revascularization.
Angiographic analyses
The randomized quantitative coronary angiography (QCA) subset included 2178 non-diabetic patients, 469 diabetic patients requiring oral medications only, and 216 insulin-treated diabetic patients. Results for the pooled groups are presented in Table 5.
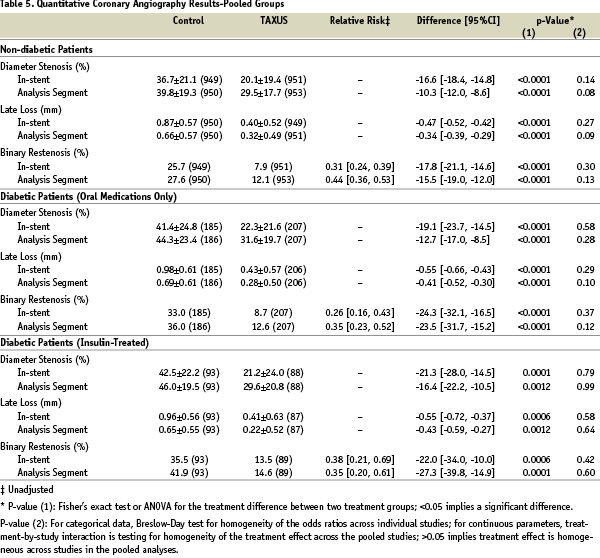
As expected, diabetic patients receiving BMS had worse angiographic outcomes than did non-diabetic patients. Among non-diabetic patients receiving TAXUS versus BMS, binary restenosis in-stent and in-segment decreased 69% and 56% (p<0.0001), respectively. This beneficial effect on restenosis was sustained among diabetic patients receiving TAXUS stents. Patients on oral medications experienced decreases of 74% and 65% in-stent and in-segment, respectively (p<0.0001), while the higher-risk insulin-treated group showed decreases of 62% (p=0.001) and 65% (p=0.0001). Likewise, the significant in-stent and in-segment late loss and percent diameter stenosis benefit associated with TAXUS that was observed with non-diabetic patients was preserved in both groups of diabetic patients (Table 5).
Discussion
The promise of a benefit from drug-eluting stents for the diabetic patient population had been suggested by trends seen from individual clinical trials with both paclitaxel and sirolimus-eluting stents which have included variable percentages of diabetic patients.
This study is the first to use an integrated analysis of randomized data to examine aggregate results in a lower frequency but higher risk diabetic population enrolled in intra-coronary stent trials. This post hoc analysis pooling 469 diabetic patients requiring oral medications only and 216 insulin-treated showed that the restenotic benefit as measured by TLR, binary restenosis, and percent diameter stenosis is extended to diabetics with maintenance of the safety profile. Diabetic disease is characterized by smaller vessel size, longer lesion length, and greater plaque burden as previously described.10 For diabetic patients, the outcomes with bare metal stents were worse compared to non-diabetic patients, as expected3,5,7,11,12,19. Use of TAXUS stents yielded statistically significant reductions in the need for repeat revascularization with a TLR rate of 7.9% for diabetic patients on oral medications, and a TLR rate of only 5.8% in insulin-treated patients. Additionally, the results reported here represent the first time that significance was achieved for binary clinical indices in the high-risk insulin-treated subgroup. These restenotic benefits were associated with a similar safety profile to that seen in diabetic patients treated with bare metal stents and were also demonstrated in the diabetic subset treated with insulin, often considered those at highest risk. No significant differences in stent thrombosis rates were observed between the various groups (TAXUS versus control, diabetic versus non-diabetic) (Table 5).
Among patients with diabetes, TAXUS in comparison with BMS significantly reduced binary restenosis rates, both in-stent (74% and 62% decrease for patients treated with oral medications or insulin, respectively) and in-segment (65% decrease for both). These results were similar to those in non-diabetic patients receiving the TAXUS stent versus the control BMS (decrease of 69% in-stent and 56% in-segment, p<0.0001). Likewise, significant reductions were seen for late loss and percent diameter stenosis among patients receiving the TAXUS stent (Table 5). Overall, the relative and absolute magnitude of the TAXUS benefit in terms of reducing clinical and angiographic restenosis was comparable between non-diabetic patients, diabetic patients treated with oral medication only and diabetic patients treated with insulin.
The advantage of using individual patient data to perform this integrated analysis, as opposed to the outcome summary from individual studies, is that the former allows for more extensive data exploration to investigate the treatment effect adjusting for patient demographic and prognostic factors.20 This approach has been used successfully in other clinical analyses.21-23 Statistical analysis of the four TAXUS trials indicated that outcomes could be pooled across stent platforms and dose formulations to generate a patient group of 3,445, which included a diabetic patient group of 814. Other approaches to studying infrequent events include meta-analyses of the mean data from reported trials in lieu of the individual data points24-26. Without the individual data points one cannot determine if differences in patient characteristics, trial definitions, or processes have introduced unacceptable degrees of heterogeneity that would limit comparisons across groups. On the other hand, the advantage of using mean data is that it can be analysed rapidly.
A number of mechanistic factors may contribute to the observed TAXUS diabetic benefit. Paclitaxel promotes polymerization of tubulin into stable microtubules, in turn inhibiting microtubule-dependent cell functions including cell signaling, cell proliferation, and migration.27,28 Insulin signal transduction occurs through the PI3-kinase pathway, in which activation of mTOR (blocked by rapamycin) and p70s6k (blocked by paclitaxel) leads to an increase in protein synthesis and restenosis.27,29-36 In early Type 2 diabetes, increased insulin levels result in increased activation of the PI3-kinase pathway and neointimal hyperplasia. In advanced disease (which is associated with insulin resistance), this pathway is shut down with consequent secondary signal transduction through the Ras/MAPK pathway, which is also known to lead to restenosis.32 Because paclitaxel can modulate cell mitogenesis independent of the PI3-kinase pathway, its consequent effect on insulin-dependent and insulin-independent pathways may provide increased benefit to diabetic patients.32,34,37
Recent randomized trials have compared the effect of the sirolimus-eluting Cypher® Stent (SES) versus the TAXUS® stent. In the multicentre REALITY trial (1,353 patients with 1,911 lesions), at 8 months there was no significant difference in in-lesion restenosis in the diabetic subset (p=0.44).38 The SIRTAX trial, carried out at two centres, randomized into SES (n=503) and TAXUS (n=509) arms all symptomatic patients with silent ischaemia, acute coronary syndromes, and a RVD of 2.25 mm to 4.0 mm, with no limit on the number of treated lesions or vessels or lesion size.39 At 9 months, there was a significant reduction in MACE in the SES arm among the 201 diabetic patients. Interestingly, the overall TLR rate reported for TAXUS, which was significantly higher than the SES, is inconsistent with data obtained in the TAXUS trials. In the randomized, two-center, 250-patient ISAR-Diabetes trial (125 diabetic patients per arm) a significant decrease in late loss at 6 months in the SES arm did not result in a significant difference in TLR at 9 months.40 Discrepancies between the multicentre TAXUS results reported here and those from the two-centre SIRTAX and ISAR-Diabetes
trials may arise in part due to the small number of centres, the small sample size, low rates of angiographic follow-up, inconsistencies between the SES and TAXUS groups, and the degree of independence of the core laboratories and adjudication committees. Neither SIRTAX nor ISAR-Diabetes fulfilled the recently published guidelines of the European Society of Cardiology in relation to the conduct of clinical trials in percutaneous coronary intervention.41
Historically, the use of BMS has improved angiographic and late clinical outcomes for diabetic patients when compared with standard balloon angioplasty, but late restenosis and the need for revascularization have remained significantly more prevalent among diabetic patients versus those without diabetes. Among diabetic patients receiving BMS, reported restenosis rates have ranged from 24% to 55% and rates for TLR from 16% to 24%.2-8 In this TAXUS integrated analysis, results with BMS were in line with these historical values. However, among diabetic patients treated with oral medications and receiving a TAXUS stent, the binary restenosis rate was 8.7% in-stent and 12.6% in-segment, and the TLR rate was 7.9%. Likewise, restenosis rates in the insulin-treated subset were decreased significantly to 13.5% in-stent and 14.6% in-segment with a TLR rate of 5.8%. Overall, the angiographic and clinical results of this integrated analysis suggest a significant advantage with the TAXUS stent for diabetic patients, including the high-risk insulin-treated subset. It can also be concluded from this study that there was no evidence of heterogeneity in the treatment effect (DES vs. BMS) between patients with and without diabetes.
Limitations
Among potential limitations in this study, there is some heterogeneity within the pooled groups, and the four trials defined the diabetic state differently; thus the post hoc assessment of a ‘diabetic’ was suboptimal. Differences in the prevalence of diabetes between the individual trials are likely to reflect geographic variability in the study populations. The introduction of a Type II error in the analysis cannot be excluded. Finally, while 9-month clinical data were included in the analysis, the available angiographic data were for 6-month or 9-month results. The subset comparison was confined to diabetes and other comparisons are the subject of future analysis. This is a post hoc analysis, and additional prospective comparison trials, including an adequately powered randomized controlled trial in this population, will be needed to validate the results reported here.
Conclusions
The TAXUS benefit provided for the high-risk diabetic population is in contrast to outcomes with bare metal stents which are worse for diabetic patients. The contrast between pro-stenotic forces with bare metal stents and anti-restenotic effects for diabetes receiving TAXUS suggests that paclitaxel may also block restenotic pathways unique to the diabetic milieu. For diabetic patients, especially the high-risk insulin-treated group, this could dramatically improve clinical and angiographic outcomes and offer a less invasive approach to a population with impaired wound healing.
Acknowledgments
We thank Ruth Starzyk, Ph.D. for help with the manuscript.

