Abstract
Aims: To evaluate the technical performance of a dedicated renal stent device and the clinical outcome.
Methods and results: Fifty patients with 55 renal artery stenoses (RAS) >70% (66±12 years, 58% male) were included in this non-randomised, prospective, multicentre registry. Primary endpoint was the primary patency rate at one year defined as >70% as determined by duplex ultrasound. Major secondary endpoints were procedural success, 30 days MACE rate, the impact of the intervention on renal function, blood pressure control, and on B-type natriuretic peptide (BNP) level. Procedural success rate was 100% and 30 days MACE rate was 0%. Restenosis rate (primary endpoint) and target lesion revascularisation rate after 12 months were 3.5% and 1.8%, respectively. After one year estimated glomerular filtration rate increased from 51±26 ml/min to 61±28 ml/min (P=0.004). Mean ambulatory blood pressure was reduced from 102±14 mmHg to 93±9 mmHg (P=0.001). Mean daily dose of antihypertensive drugs decreased from 3.0±1.7 to 2.7±1.4 (P=0.09). Mean BNP decreased from 251±282 pg/ml to 188±219 pg/ml (P=0.046) before discharge.
Conclusions: Technical outcome of the tested device is favourable. The impact of the stent revascularisation on renal function and blood pressure control was promising.
Introduction
Atherosclerosis is the most common cause of renal vascular disease (RVD)1,2. Stent-angioplasty is established as the first treatment to be proposed for most patients who have symptomatic RVD2-4. The impact of renal angioplasty on renal function and hypertension is still a matter of debate1,5; randomised controlled trials comparing the clinical outcome of stent-angioplasty and best medical therapy are still ongoing or close being started.
The PRECISION registry (Renal Artery Angioplasty in Patients with Renal Insufficiency and Hypertension Using a dedicated Renal Stent Device: non-randomised, prospective, multicentre evaluation of the Hippocampus™.014 Balloon expanding rapid exchange renal stent system for atherosclerotic renal artery stenosis), a non-randomised, prospective, German multicentre clinical registry was designed as a pilot study to investigate the performance of a new renal stent device, which could be later used in a randomised study comparing stent angioplasty of renal artery stenosis (RAS) with best medical therapy. The primary study endpoint of this anticipated study could be the difference in outcome of estimated glomerular filtration rate (eGFR) after one year and the outcome data of the present registry should be used for the calculation of the study sample size. Furthermore, the impact of revascularisation on the brain natriuretic peptide (BNP) value, which was recommended as predictive marker for improvement of blood pressure control in a recent publication6 should be investigated in a subgroup of the entire patient cohort.
Methods
Patients
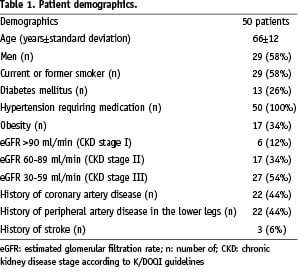
This non-randomised, investigator initiated prospective clinical study was conducted at three German study sites. Patient recruitment lasted from June 2005 to June 2006 and 1-year follow-up was completed in July 2007. A total of 128 patients had been screened during the recruitment period. Seventy-eight patients either refused study participation or had to be excluded failing to meet the study inclusion criteria. Finally, according to the study protocol, 50 patients with 55 atherosclerotic renal artery stenoses of at least 70% diameter stenosis confirmed by angiography were included into the study. Mean age of the study population was 66±12 (range 41-88) years, 58% (29/50) patients were male. The patient baseline characteristics are given in Table 1, the lesion characteristics in Table 2.
Key inclusion criteria included patients having an atherosclerotic RAS with an at least 70% angiographic diameter stenosis by visual estimation, a reference target vessel diameter of 4.0 mm to 7.0 mm, patients having hypertension and/or renal insufficiency, and a baseline serum creatinine concentration <4.0 mg/dL.
Key exclusion criteria were patients having non atherosclerotic RAS, RAS <70% as determined by angiography, and baseline serum creatinine concentration > 4.0 mg/dL.
Study conduct
Work-up before the intervention and before discharge included blood sample analysis including, besides others, red and white blood cell count, thrombocyte count, coagulation parameters, BNP (subgroup of 29 patients included at the Herz-Zentrum Bad Krozingen), serum creatinine and urea concentration, ambulatory blood pressure measurement, renal duplex ultrasound examination, measurement of body weight and height, analysis of drugs, and in a subgroup of patients, echocardiography.
Following the index procedure, all patients underwent follow-up examinations at one, six and 12 months including duplex ultrasound, analysis of medication, measurement of serum creatinine concentration, calculation of the eGFR, and recording of a 24-hour ambulatory blood pressure measurement. Forty-five (45) of the 50 patients (90%) underwent the 12 months follow-up examinations, one patient died (2%) and four patients (8%) were lost for follow-up. The study was approved by the institutional ethics committees of the participating centres. All patients signed written informed consent.
Definitions
The primary endpoint was the primary patency rate at 12 months defined as diameter stenosis <70% as determined by duplex ultrasound7. The major secondary endpoints were procedural success, the 30 days major adverse event rate (MACE), the impact of the intervention on renal function including serum creatinine concentration, eGFR and serum urea concentration, on blood pressure control (ambulatory blood pressure measurement recordings documenting systolic, diastolic and mean arterial blood pressure), and on the BNP value determined at baseline and at least 48 hours after the intervention, and finally the predictive value of a baseline BNP cut-off value of 80 pg/ml on the blood pressure outcome.
Procedural success was defined as a residual stenosis <30% by visual estimation following stent placement. Definition of in-stent stenosis was based on a side difference of intrarenal resistance index (RI) >0.05 representing an angiographically confirmed >70% restenosis in unilateral restenosis and an acceleration time >0.07 sec in bilateral restenosis7.
According to the guidelines of Good Clinical Practices (GCP), EN540, EU Directive 20/2001/CE, Declaration of Helsinki and local regulations, the study was independently monitored and audited by Krauth Medical, Hamburg, Germany.
Improved renal function was defined as an increase in eGFR >2 ml/min, stable renal function as an eGFR of±2 ml/min, and a deterioration of renal function as a decrease in eGFR of >2 ml/min after one year compared to baseline.
Improved blood pressure was defined as a decrease in mean blood pressure >5 mmHg, stable blood pressure as a mean blood pressure of±5 mmHg, and deteriorated blood pressure control as a mean blood pressure of >5 mmHg after one year compared to baseline.
Each antihypertensive drug with its individual dose taken at baseline was counted as one daily dose. After one year, every increase in dose was counted as 1.5 daily doses, every reduction in dose was counted as 0.5 daily doses. Every additional drug was counted as one daily dose.
Statistics
The statistical analyses were performed using the SPSS/PC (version 13.0, SPSS Inc., Chicago, IL USA) software package. A statistical significance level of 0.05 was used. Comparisons were made using analysis of variance for independent samples by t-test and chi-square tests for binary events as appropriate. All hypothesis testing was two-tailed (paired t-test). Cox Proportional-Hazards regression analysis was used as the appropriate method throughout. Restenosis free survival rates were calculated with the use of the Kaplan-Meier method.
Treatment technique and device description
All interventions were performed using the guiding catheter technique with 0.014 inch guidewires. Engagement of the renal artery was managed either using a 5 Fr diagnostic catheter (telescope technique) or directly using a 6 Fr guiding catheter with varying shape.
The Hippocampus™.014 Balloon Expanding Rapid Exchange Renal Stent System (Invatec Corp., Concesio Brescia, Italy) device consists of a long “non-flip-tip” with progressive flexibility and minimal entry profile, a 6 folded high pressure balloon, and a tight FIXä secure crimping of the stent. Since the system shows a progressive flexibility, coming from the long tip, followed by the long balloon-cone, the guidewire will not be straightened and possibly flipped out of the origin (Figure 1).
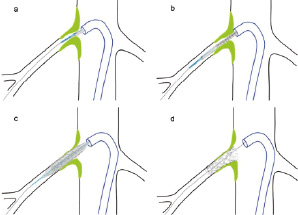
Figure 1. Device application showing the long anti-flip tip (a) with progressive flexibility and minimal entry profile. As soon as the balloon segment with the crimped stent is advanced through the curve of the guiding catheter the long tip is already inserted in the ostium so the position can not be lost again (b). (c) Positioning of the stent covering the ostial plaque, (d) result after stent placement with a slight (1-2 mm) protrusion of the stent into the aortic lumen.
As soon as the balloon segment with the crimped stent is advanced through the curve of the guiding catheter or introducer sheath, the long tip is already inserted in the renal artery origin so the position can not be lost again. The stent itself shows a progressive radial strength towards the proximal end which covers the renal artery origin.
Medication
All patients received dual antiplatelet therapy started at least one day before the index procedure: aspirin 100 mg per day for life and clopidogrel 75 mg per day for four weeks after a loading dose of 600 mg. During the procedure, 2500 to 5000 IU of heparin was given intra-arterially after sheath placement. Anticoagulation was discontinued after the intervention.
Thirty-nine patients (78%) received angiotensin-converting-enzyme inhibitors or angiotensin-1 receptor antagonists, and again 39 patients (78%) took lipid lowering drugs during the follow-up period.
Results
Technical results
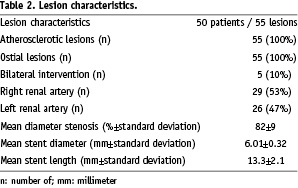
In 45 patients unilateral RAS (90%) and in five patients bilateral RAS (10%) was treated resulting in a total of 55 lesions. The right renal artery was affected in 29 cases (53%), the left renal artery in 26 cases (47%). Angiographic mean diameter stenosis by visual estimation was 82±9% (range 70 - 95%), mean stent diameter was 6.01±0.32 mm (range 5 to 7 mm) and mean stent length was 13.3±2.1 mm (range 10 to 20 mm). Procedural success rate was 100% with a reduction of residual diameter stenosis <30% in all cases leading to a mean residual stenosis of 4±3% (range 0% to 20%, Table 3).
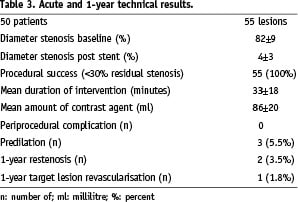
All interventions were performed via a femoral access. Predilation with an undersized balloon of the lesion was performed in three tight stenoses (5.5%), direct stenting in the remaining 52 lesions (94.5%). In two lesions, two overlapping stents had to be deployed due to incomplete lesion coverage. Mean intervention time was 33±18 min (range 11 to 75 min). In all interventions an isosmolar contrast agent (270 I/ml or 300 I/ml) was used with an average of 86±20 ml (range 30 to 230 ml, Table 3). No peri-interventional complications occurred. Restenosis rate (primary endpoint) diagnosed by duplex ultrasound and verified by angiography after 12 months was 1.8% (1/55 lesions).
Clinical results
The 30 days MACE rate was 0% and target lesion revascularisation rate 1.8% (1/55).
Serum creatinine concentration was reduced from 1.40±0.60 mg/dl (range 0.6 to 3.1) at baseline to 1.27±0.58 mg/dl (range 0.6 to 3.2) after one year (P=0.001; Figure 2), estimated glomerular filtration rate increased from 51±26 ml/min (range 18 to 134) at baseline to 61±28 ml/min (range 14 to 122) after one year (P=0.004; Figure 3).
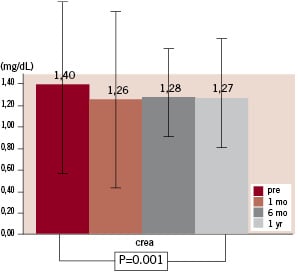
Figure 2. Course of serum creatinine concentration.
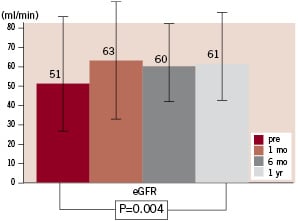
Figure 3. Course of estimated glomerular filtration rate (eGFR).
GFR increased >2 ml/min in 59.5%, was unchanged (±2 ml/min) 21.5% and decreased >2 ml/min in 19%. Serum urea concentration was unchanged after 1 year compared to baseline (52±19 mg/dl vs. 52±25 ml/dl, P=0.82).
Systolic and diastolic mean blood pressure values dropped from 148±17 mmHg and 78±10 mmHg at baseline to 133±15 mmHg and 72±9 mmHg at one year, respectively (P=0.001 each); the average of mean ambulatory blood pressure was reduced from 102±14 mmHg (range 56 to 135) to 93±9 mmHg (range 68 to 114) after 1 year (P=0.001; Figure 4).
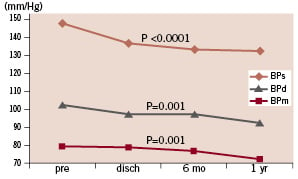
Figure 4. Course of average systolic, diastolic, and mean blood pressure.
Sixty-three point six percent of the patients had a decrease in mean blood pressure of >5 mmHg, 33.2% were stable in the range of±5 mmHg, and 3.2% had an increase of >5 mmHg (3%). Mean daily dose of antihypertensive drugs decreased from 3.0±1.7 to 2.7±1.4 (P=0.09).
Mean BNP decreased from 251±282 pg/ml (range 5 to 1300) at baseline to 188±219 pg/ml (range 10 to 758) at discharge (P=0.046, Figure 5a). A BNP value >80 pg/ml at baseline was associated with a drop of average mean blood pressure of >5 mmHg after one year compared to baseline in 84.6% of the patients whereas this decrease was found only in 58.3% of the patients with a BNP level <80 pg/ml at baseline. In those patients who experienced a reduction in mean blood pressure >5 mmHg average BNP value was 202±240 pg/ml (range 5 to 758) whereas it was 85±89 (range 11 to 260) in patients without adequate blood pressure response (P=0.2, Figure 5b).
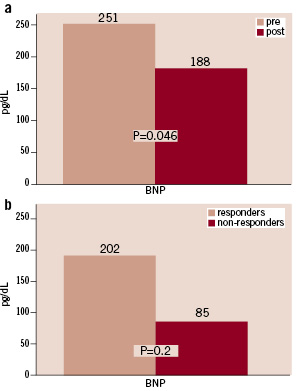
Figure 5. a) Brain natriuretic peptide (BNP) at baseline compared to at least >36 hours after the procedure. b) BNP values for blood pressure responders and non-responders defined as a decrease in mean blood pressure >5 mmHg.
Figure 6 shows the Kaplan Meier curve for restenosis and TLR free survival.
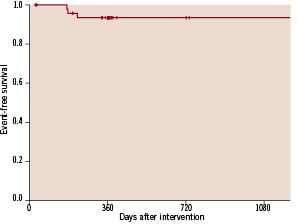
Figure 6. Kaplan Meier curve for restenosis and TLR free survival.
Discussion
This multicentre prospective controlled evaluation of the technical and clinical performance of a new stent device dedicated to the treatment of RAS gives more evidence about the beneficial effect of stenting severe RAS on blood pressure control and preservation of renal function. Moreover, the tested stent device documented a superior 1-year patency rate compared to previous reports with other stent devices even if it has to be taken into account that the cut-off values for the definition of restenosis following stent-supported angioplasty are not uniform throughout the literature8-12.
Despite using the femoral access in all interventions during this registry even in difficult anatomical situations treatment success was achieved in all cases without major complications; this result might be supported by the unique design of the tested stent-device with its long anti-flip tip. More importantly we documented a 1-year restenosis rate defined as an at least 70% stenosis by duplex ultrasound of only 3.5% which is the lowest currently reported. With first generation stent devices restenosis rates defined as either 50% or 70% ranged from 11% [9] to 21%13. Even using contemporary premounted low-profile stainless steel devices restenosis rates were reported higher compared to our study device ranging from 5.5% [11] to 14%12. Besides the stent design and the stent material (cobalt chromium) secondary preventive medical treatment might have affected the good long-term technical outcome of the study cohort. The majority of the patients received angiotensin receptor blockers or angiotensin converting enzyme inhibitors (78%) and / or cholesterol lowering agents (78%) according to the recent guidelines for secondary prevention in cardiovascular diseases14. Furthermore, all patients uniformly were treated with dual antiplatelet therapy during a 4-week period following the intervention. Even considering the relatively low number of lesions included into the present study the long-term performance of the stent-device is remarkable and probably one cause for the persistent improvement of blood pressure control and renal function after one year in this study cohort.
Blood pressure control could be improved significantly in the entire study cohort. Only 3% of the patients showed a deterioration of blood pressure control after one year according to the study definitions. This result is in line with previous single centre reports8,13,15 and the US multicentre registry report on the 1-year outcome of blood pressure control following stenting of at least 70% RAS10.
The present study shows a significant increase in eGFR of 10 ml/min which is similar to the increase of 15 ml/min in the DRASTIC trial for the balloon angioplasty arm16. After one year, 19% of the patients showed a deterioration of renal function defined as a decrease in eGFR >2 ml/min. In the remaining 81%, renal function was stabilised or – even better – improved. Considering the currently upcoming and industry pushed discussion on the role of distal protection devices in renal artery intervention17,18 it has to be emphasised that all interventions in this study were performed without the use of protection devices.
One of the secondary endpoints was to test the hypothesis that a baseline BNP value higher than 80 pg/ml might be predictive for an improved blood pressure control following the intervention as suggested by Silva et al6. Insignificantly more patients (86.7%) with a BNP level of >80 pg/ml at baseline finished the study with an improved blood pressure control whereas this amelioration was seen only in 58.3% of the patients with a pre-interventional BNP value <80 pg/ml. The BNP is affected by several cardiovascular covariables; it seems therefore unlikely that this parameter will allow a reliable patient screening method in an unselected patient cohort in terms of prediction of treatment success with regard to improved blood pressure control following renal artery revascularisation. Proper patient selection in terms of indicating endovascular treatment only in at least 70% renal artery stenosis might be a more appropriate screening tool.
In summary this multicentre clinical registry gives more evidence for the beneficial clinical impact of RAS revascularisation regarding blood pressure control and preservation of renal function in a properly selected patient cohort. Furthermore, combined with a guideline conform secondary preventive medical therapy, the tested stent device shows a favourable acute and long-term performance.
The major limitation of this study is the small sample size and the lack of randomisation. Both factors are affected by the very nature of this study which was designed as a pilot study for an upcoming multinational randomised controlled trial comparing stent revascularisation combined with best medical therapy and best medical therapy alone (the RADAR trial).
Appendix
Participating centres: Department Angiology, Herz-Zentrum Bad Krozingen, T. Zeller (PI); Universitäres Herz- und Gefäßzentrum Hamburg, H. Krankenberg; Department Radiology, Universitätsklinik Kiel, S. Müller-Hülsbeck.

