Description and history
Extensive clinical practice supported with evidence gathered during numerous animal studies has shown that in addition to its excellent biocompatibility, hydroxyapatite (HAp, a crystalline derivative of calcium phosphate) is non-toxic and does not induce allergic or inflammatory reactions. The latest research on HAp appears to confirm that HAp crystals “do not acquire a pro-inflammatory or thrombogenic phenotype, but express markers of functioning endothelium that might contribute to angiogenesis.”1 The new generation of non-polymeric drug-eluting cardiovascular stents (as compared to polymer-coated stents) is expected to offer drug delivery solutions which will be less thrombogenic and more tolerable by the body environment (less restenotic) while, at the same time, capable of delivering the desired quantity of drugs in a manner that reflects recommendations of scientists, and responding to the real-life needs and abilities of the patient. These generic observations prompted MIV Therapeutics Inc. to consider HAp as a coating suitable for drug delivery on cardiovascular stents. Such HAp coated stents are being developed within the collaborative research and development program between University of British Columbia, and MIV Therapeutics Inc., both located in Vancouver, BC, Canada. The challenge is that the brittleness of ceramic HAp coating has to withstand deformation during stent crimping and implantation, and remain undamaged and biologically inert years after the implantation into arteries of human heart. In response to this challenge, several novel coating technologies have been developed in 2003-2005 for deposition of HAp ceramics on stents and other implantable medical devices, by combined efforts and ingenuity of the teams of MIV Therapeutics, Inc. and University of British Columbia.
Technical specifications
“Wet” chemical technologies were used for deposition of ultra-thin (0.1-0.2 µm) and micro-thin (0.3-1.0 µm) HAp coatings. The ultra-thin films composed of high-density non-porous hydroxyapatite are designated primarily as a biocompatible surface modification of metallic implants, whereas the micro-thin films which possess highly porous structure were developed and evaluated also as a potential vehicle for effective and highly engineerable drug delivery. The deposition of the 0.1-0.2 µm ultra-thin films of HAp ceramics used sol-gel (SG) subjected to heat treatment at 450°C which ensured their structural integrity and necessary mechanical properties. The resulting SG-HAp Class “0” ultra-thin “passive” (drug-free) coatings are very well bonded to metal substrates such as stainless steel or Co-Cr alloys, feature acceptably smooth surface (Figure 1) and survive undamaged the stent crimping-implanting-expansion procedures and demanding fatigue life evaluation which extended to 40 mln cycles of real-life simulating evaluation in artificial arteries.

Figure 1. Ultra-thin hydroxyapatite sol-gel coated 316L stainless steel stent, seen under optical microscope (left, as-coated) and scanning electron microscope (right, after crimping and expansion of the stent). The characteristic “rainbow” effect seen under the optical microscope is indicative of the very small thickness of the coating (~200nm), slight variation of the coating thickness across the stent surface. The crimping-expansion test does not affect the coating.
The SG-HAp passive film equipped with excellent mechanical properties and long-term durability of its highly crystalline coating can also serve as a nucleation film for additional layers of functionally graded, thicker (0.3-1.0 µm) nanoporous coatings designated for drug encapsulation and controlled release (Figure 2).

Figure 2. Hydroxyapatite coating applied with electrochemical deposition (ECD), as seen under SEM. Note nano-porous structure of the coating seen under high magnification (right).
For deposition of the second layer highly-porous coating suitable for drug delivery purposes through applications on coronary stents and other implants, electro-chemical deposition (ECD) process has been developed by the MIV Therapeutics, Inc. and University of British Columbia team. The electrolyte solution used for the ECD-HAp consisted of 0.042 mol Ca(NO3)2 and 0.025 mol NH4H2PO4 in distilled water. The pH of the solution was approximately 4.2, and the solution temperature was maintained at 65°C. The ECD was carried out galvanostatically at a cathodic current density of 0.6 mA/cm2, the specimen was then rinsed with distilled water and air dried. Depending on deposition time and other process parameters, thickness of the coatings was 0.3-1.0 µm range, and porosity in 40-60 vol% range (Figure 2).
Two classes of such nano-porous HAp coatings for drug delivery are offered. Class 1 coating, 0.3-0.5 µm thick, capable of delivering of 20-40 µg of a drugs such as sirolimus or paclitaxel from a 16 mm long stent, through leaching directly from the nano-pores of HAp film. These Class 1 coatings are still thin enough to allow crimping-implanting-expansion procedures with no damage to the coating. Class 2 coatings are 0.5-1.0 µm, or thicker, and are designed for delivering larger amounts of drug (>50 µg for 16mm stent), for an extended period of time. Class 2 coatings are too thick to be used in the pure form of HAp. Therefore, to obtain Class 2 coatings, the 0.5-1.0 µm thick nano-porous HAp film is first impregnated with the drug, followed with deposition of a membrane-film of bioresorbable polymer such as PLGA, Figure 3.

Figure 3. Composite coating applied with electrochemical deposition (ECD) of hydroxyapatite combined with biopolymer filler, seen under high-magnification electron microscopy.
The porous HAp coatings were impregnated with 3-10wt% PLGA solution. The weight of HAp coatings loaded with drugs and impregnated with polymer on 16mm stents was in excess of 130 µg. Whereas the polymer does not mix or otherwise interfere with the drug film within the nano-pores of HAp, it provides (i) toughening of the ceramic HAp film, and (ii) a restrain barrier for slower release of the drug. The resulting PLGA-HAp matrix composites combine the reinforcing polymer phase with the ceramic matrix phase to create coatings with new and superior properties to both pure ceramic or pure polymer coatings. The HAp matrix provides a stable, biocompatible nano-porous framework with relatively high capacity for drug. The biopolymer filler provides toughness to the ceramic, and also adds more controls of drug delivery. The novel composites combine the most desirable properties of bioceramics with those of biopolymers, to tailor properties such as strength and elasticity to meet the specific system requirements (Figure 3).
Pre-clinical experience
In 2004, MIV’s ultra-thin “passive” non-drug-eluting HAp stent coatings have begun in-vivo tests. A pilot study in rabbits has offered an early glimpse of safety when no significant difference in neointimal formation was found after 4 weeks following implantation of HAp-coated stents in iliac arteries in comparison to bare metal stents of identical design (data on file with MIV Therapeutics). This paved the way to a more formal testing in porcine coronary arteries, involving specialized device-oriented tissue processing (plastic embedding) and expert pathology evaluation. A pilot study was conducted testing 4 versions of HAp coating differing in the mode of deposition, porosity and, consequently, thickness. Three to five stents per coating type were utilized and compared to the bare metal stent of the same design at 28 days of follow-up. All versions of coating confirmed expected high biocompatibility in this limited experience, and the version with least neointimal growth was selected for future development and testing in a larger scale study with a longer follow-up. Subsequently, in 10 miniswine, 20 coronary arteries were randomized to receive a HAp-coated 16mm Millennium Matrix stent or an identical bare metal stent oversized to 110% of arterial diameter and followed for 3 months. At termination, angiography and intravascular ultrasound were performed, followed by harvesting arteries for histology. At 3 months, HAp-coated stents featured nearly identical % late lumen loss (21±11%) as bare metal stents (22±13%) in the quantitative angiographic analysis and mean Percent Area Stenosis in intravascular ultrasound (HAp: 26±10% vs. bare metal stent: 26±9%). In light microscopy, HAp-coated stents showed exemplary biocompatibility, on par with their bare metal controls. Low injury and inflammation parameters, complete endothelialization and low neointimal growth were uniformly found (Table 1).
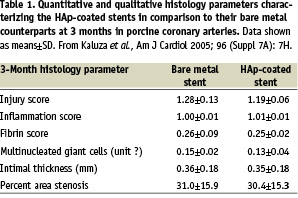
Based on these 3-month data, it was concluded the microporous ultra-thin HAp coating with a potential for drug elution showed promise of excellent long term biocompatibility and made an attractive candidate for next-generation stent coating.2
Future directions
Currently, a number of in-vivo porcine studies are ongoing, testing different variants of HAp and composite coating, including drug-eluting ones. These in-vivo studies complement intense ongoing laboratory efforts by MIV Therapeutics to further enhance the composite and polymer-free coatings for optimal drug delivery. If confirmed by large scale animal studies, hydroxyapatite coatings have the potential to offer a truly superior, biologically inert, uniquely biocompatible (derived from material naturally occurring in the human body) platform for drug elution on cardiovascular stents and other implantable medical devices.

