Abstract
Aims: We hypothesised that ischaemic preconditioning (IP) results from complex cellular mechanisms without significant collateral recruitment or clinical pre-intervention interference.
Methods and results: A total of 58 patients underwent three 2-min balloon inflations separated by 5-min reperfusions. Anginal symptoms were graded according to this scale: 0 = absent, 1 = mild, 2 = moderate and 3 = severe. ST-segment shift and QT dispersion (QTd) were measured from 12 lead ECGs. Ejection fraction (EF) was assessed by 2D echo and collateral flow recruitment by collateral flow index (CFI). Anginal scores were 2.4±0.6, 1.7±0.5 and 1.2±0.6 (p<0.05); ST-segment shifts were 6.0±2.1, 3.8±1.8, and 1.9±1.2 mm (p<0.05); QTd increased from a baseline value of 39±24 to 96±27 (p<0.05) and decreased to 66±23 and 45±16 ms, at the end of the first, second and third inflation, respectively. EF decreased from a baseline value of 63±3% to 33±2%, 34±3%, and 36±5% in the three inflations. The CFI was approximately 0.15 in all ischaemic periods.
Conclusion: These results suggest that IP does occur during repeated brief coronary artery occlusion in patients with a low likelihood of both collateral recruitment and clinical pre-intervention interference.
Introduction
Ischaemic preconditioning (IP) is a process by which a brief ischaemic episode confers a state of myocardial protection against subsequent sustained periods of ischaemia.1 The mechanism of this endogenous phenomenon is at present unknown, though recent research findings suggest a major role of the mitochondria.2-4 Also, IP involves mutual triggers, mediators, and complex second messenger pathways that seem to have such components as adenosine, adenosine receptors, the epsilon isoform of protein kinase C, the ATP-dependent potassium channels, and others.5-7 A paradoxical protective role of oxygen radicals is involved as well.5-7 On the other hand, among patients with coronary artery disease (CAD), about one third have functionally sufficient coronary collaterals that are able to prevent signs of myocardial ischaemia during brief vascular occlusions, and coronary lesion severity is the only independent pathogenetic variable related to collateral flow.8-9 Nevertheless, even in the absence of obstructive CAD, collateral flow to a briefly occluded coronary artery has been found that is sufficient to prevent anginal chest pain and ECG signs of myocardial ischaemia in about one quarter of the population studied.10 Therefore, an absence of anginal symptoms and ECG signs of myocardial ischaemia in patients with and without CAD, not only during the first vascular occlusions but also during the brief sequential ones, indirectly represents well-developed collateral protective against ischaemic insult.11 Accordingly, in the absence of collateral recruitment, that preconditioning might occur in humans is suggested by the findings that repetitive brief occlusions in the coronary artery are associated with gradually decreasing anginal pain, ST-segment shift, lactate production, QT dispersion (QTd), defence offered by pre-infarction angina, and anginal warm-up phenomenon.5-7,12-18 This is also suggested by investigations carried out on human cardiac biopsies that demonstrate metabolic properties indicative of IP.19
Coronary angioplasty (PTCA) provides an exceptional opportunity to study the response of the human myocardium to brief periods of controlled ischaemia and reperfusion. Previous PTCA studies12,20-23 that showed a clinical preconditioning effect could not completely exclude the significant involvement of collateral circulation or otherwise24, concluded that the observation of ischaemic tolerance in the clinical setting was attributable, in part to enhanced collateral circulation. More importantly, no studies have consistently screened patients with the possibility of collateral recruitment, and at the same time, eliminating the major pre-intervention interference to preconditioning. Also, it is essential to mention that although 120-150 seconds of coronary artery occlusion during PTCA studies frequently elicits preconditioning21,24-25, the response to inflations of 90 seconds period are unpredictable with both positive12,20,23 and negative26 results. In addition, many drugs have been identified that produce a reduction in or induction of indices of preconditioning during PTCA.27 The aim of the present investigation was to assess the preconditioning in patients selected on the basis of clinical and angiographic characteristics indicative of a very low likelihood of both collateral recruitment and clinical pre-intervention interference.
Methods
The study was performed according to the prescriptions of the Helsinki declaration (revised version of Somerset West, Republic of South Africa). Also, the present investigation was approved by the scientific committee and the committee on ethics of the Heart Institute (InCor), University of São Paulo Medical School, Brazil.
Study population
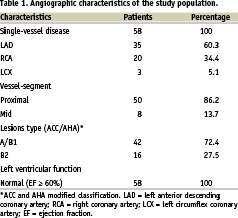
The study included 58 patients (37 men, 21 women; mean age 67 years, ranging from 51 to 75 years) with stable coronary syndrome and normal left ventricular function, and who underwent elective PTCA in lesions with less than 70% diameter stenosis by quantitative coronary angiography (QCA). All patients had single-vessel disease, and the interval between the last episode of angina and PTCA was 61±12 days. Patients who matched one of the following criteria were not included in the study: previous Q-wave myocardium infarction, mild or severe left ventricular dysfunction, lesions with mean diameter stenosis greater than 69%, coronary artery multivessel disease, presence of visible collateral circulation to the target coronary artery at the time of diagnostic angiography, use of drugs known to affect ischaemic preconditioning or QT interval, history of coronary artery bypass grafting, established atrial fibrillation, left or right bundle branch block, severe or poorly controlled hypertension, history of sustained ventricular tachycardia or of ventricular fibrillation, a less than 1 month interval between last episode of chest pain and PTCA and diabetes mellitus. Table 1 outlines the angiographic features at the time of diagnostic angiography.
Study protocol
PTCA was performed using the femoral approach. A 7Fr guiding catheter was advanced through the femoral artery. Baseline heart rate, aortic pressures, surface 12-lead ECGs, and contrast echocardiograms were performed before coronary angiography. An interval of at least 5 min was allowed for dissipation of the effect of the ionic contrast medium on coronary flow velocity and vasomotion. No intracoronary or sublingual nitroglycerin was given before or during the study protocol. The guide-wire was positioned distal to the stenosis to be dilated. Patients were submitted to three 2-min balloon inflations separated by 5-min reperfusion periods (during which the balloon was deflated and withdrawn proximal to the lesion with the guide wire remaining across the lesion). For each patient, the balloon pressure was the same (around 6 atm) during the three inflations. The balloon-to-artery diameter ratio was undersize approximately in 0.5 mm for all patients. After the last inflation/deflation, a stent was implanted in the target vessel depending on the specific needs of individual patients. Four methods were utilised to characterise myocardial ischaemia during balloon inflation/deflation: anginal chest pain, ST-segment shift in surface 12-lead ECGs, QT interval dispersion, and left ventricular wall motion. Anginal pain was assessed using a semi-quantitative scale (0 = no angina; 1 = mild chest discomfort; 2 = moderate anginal chest pain; and 3 = severe anginal chest pain). ST-segment depression was measured to the closest 0.5 mm, 0.08 s after the J point, and ST-segment elevation was measured to the closest 0.5 mm, 0.02 s after the J point. ST-segment shifts were measured in the lead that displayed the greatest amount of ST deviation during the first balloon inflation. The QT interval was measured from the onset of the QRS complex to the end of the T wave, i.e. return to the T-P baseline. When U waves were present, the QT interval was measured to the nadir of the curve between the T and U waves. When the end of the QRS complex or T wave could not be identified, the lead was not included in the analysis. At least three precordial leads and a minimum of seven leads were required for quantification of the QT dispersion (QTd). The QTd was defined as the difference between the maximum and the minimum QT values occurring in any of the 12 ECG leads. The left ventricle (LV) was divided according to the recommendations of the American Society of Echocardiography and a wall motion score index (WMSI) was assigned on the basis of the 16-segment model.28 LV end diastolic volume index, end systolic volume index, and LVEF were measured at four-chamber view by the modified Simpson´s rule method,29 at each stage. A second-generation contrast agent (PESDA-Perfluorocarbon Enhanced Sonicated Dextrose and Albumin) was used in order to optimise the endocardial border delineation since the left lateral position was suboptimal in most patients. All measurements were made by consensus of two experienced echocardiographers blinded to the stages of the study protocol.
Coronary collateral assessment
Examinations were performed using a pressure guide from Radi Medical®, Uppsala, Sweden. Coronary collateral function was estimated through the pressure-derived collateral flow index (CFI, no units). The intracoronary pressure-derived CFI was determined by simultaneous measurement of the mean aortic pressure (Pao, in mm Hg), using the angioplasty guiding catheter and the distal coronary artery pressure during balloon occlusion (Poccl, in mm Hg), using the fiberoptic pressure monitoring guide wire. The central venous pressure (CVP) was estimated to be equal to 5 mm Hg. The CFI was calculated as (Poccl - CVP) divided by (Pao - CVP). The CFI is a measure of the collateral flow relative to the normal flow through the patent vessel. The CFI was determined at the end of each balloon inflation period and a value of >0.25 was empirically9,30-33 considered to represent well-developed collaterals protective against myocardial ischaemic insult during each coronary occlusion.
Statistical analysis
Categoric variables were expressed as frequencies. Continuous variables were expressed as mean±SD. Statistical analysis of the values of the anginal score, ST-T segment shift, QTd, LVEF, WMSI, and CFI during the different phases of the protocol was performed using repeated measures analysis of variance. Univariate analysis was performed using the Fischer exact test for categoric values and the Student t test or the non-parametric Mann-Whitney and/or Friedman test for continuous variables. p < 0.05 was considered statistically significant.
Results
Coronary angioplasty
The diameter stenosis ranged from 61% to 69% (mean = 65%±4%). In every patient the chest pain had disappeared and the ST-T-segment on the surface ECGs had returned to within 1 mm of baseline before the second and third balloon inflations. PTCA was successfully performed in all 58 patients, and there was no electrocardiographic evidence of myocardial injury or post-procedural marker elevation in any patients at the end of the protocol. There were no significant changes in heart rate from the control state to the first, second, and third balloon inflations and deflations, but there was a significant reduction (p <0.05) in mean arterial blood pressure from 108±9 mm Hg at baseline to 97±8 mm Hg at the end of the first reperfusion period. However, there were no further significant changes in aortic pressure till the end of the protocol.
Symptoms during repeated coronary occlusion
All 58 patients developed anginal symptoms during the first inflation. Overall, the severity of chest pain was significantly (p <0.05) greater during the first inflation and gradually decreased during the second and third inflations (scores 2.4±0.6, 1.7±0.5 and 1.2±0.6, respectively). So, the intensity of the anginal pain was significantly (p <0.05) less during the second and especially the third inflations than during the first.
ECG changes during repeated coronary occlusions
Before each inflation, there were no ST-segment shifts on 12-lead ECGs in any patient. All 58 patients exhibited ST-segment shifts during balloon inflations (ST-segment elevation in 56 patients and depression in two patients – both with lesions in the left circumflex coronary artery). The shift was significantly (p <0.05) greater during the first balloon inflation than during the second and the third inflations. The mean ±SD values for ST shifts during the first, second, and third inflations were 6.0±2.1, 3.8±1.8 and 1.9±1.2 mm, respectively, p <0.05. Consequently, the magnitude of the ST-segment shift was significantly (p <0.05) less during the third inflation than during the first and second inflations.
QT dispersion during repeated coronary occlusion and reperfusion
All 58 patients had adequate data for analysis of QTd. The maximal QT interval measured was 478±15 ms (ranging from 466 to 509 ms) and occurred in a precordial lead in 32 patients (55.1%) and in a limb lead in 26 patients (44.8%). The minimal QT interval measured was 403±29 ms (ranging from 364 to 431 ms), occurring in a precordial lead in 26 patients (44.8%) and in a limb lead in 32 patients (55.1%). The mean values of QTd were 39±24 ms at baseline, and 96±27, 66±23, and 45±16 ms, at the end of the first, second and third 2-min balloon inflations, respectively and 86±23, 63±24, and 42±22, at the end of the first, second, and third 5-min reperfusion periods, respectively. So, the QTd increased significantly (p < 0.05) during both ischaemia and reperfusion in the first balloon inflation/deflation and there was a progressive decrease in the QTd with increasing number of balloon inflations and deflations. Therefore, there was no significant difference between the QTd at baseline and after the third balloon inflation/deflation. The QTd increased on shortening the minimal QT interval during balloon inflation and on lengthening the maximal QT interval during reperfusion.
Echocardiographic changes during repeated coronary occlusion
At baseline, normal wall motion was seen in all segments (EF = 63±3% and WMSI = 1.00). During each inflation, wall motion severely deteriorated and manifested akinesia or dyskinesis in all patients (p <0.05 baseline versus all inflations), seen from the following: The EF decreased from a control value to 33±2%, 34±3% and 36±5% in the first, second, and third inflations, and the WMSI increased from a control value to 2±08, 2±06, and 2±10 in the first, second, and third inflations, respectively. There were no significant differences among all three balloon inflations. In all cases, there was complete resolution of wall motion abnormalities before each balloon inflation.
Collateral flow index
All CFI was measured at the end of each ischaemic period, and did not differ significantly among the first, second, and third balloon occlusions, mean = 0.15±0.5, 0.15±0.7, and 0.15±0.9, respectively (ranging from 0.09 to 0.19 in all patients) in spite of progressive reductions in the anginal pain score, in the ST-segment shift, and in the QTd (Figure 1).
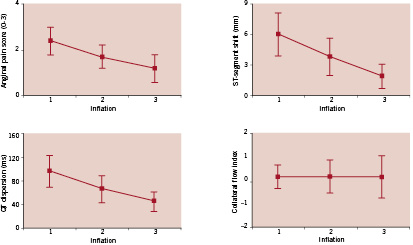
Figure 1. There were significant differences (p < 0.05) among one vs. two, two vs. three, and one vs. three balloon inflations for angina pain score, ST-segment shift and QTd. There were no significant differences among one vs. two, two vs. three, and one vs. three balloon inflations for CFI. Top: anginal pain score (left) and ST-segment shift (right). Bottom: QT dispersion, (QTd) (left) and collateral flow index (CFI) (right). Solid symbols represent means ±SEM.
Discussion
This study is the first in which the effects of repeated coronary occlusions during PTCA were analysed in patients selected on the basis of clinical and angiographic characteristics indicative of a very low likelihood of both significant recruitable collaterals and clinical pre-intervention interference to preconditioning.
All our patients had anginal symptoms, ST-segment shift, and QTd during the first 120 seconds balloon inflation and were significantly greater than during the second inflation, and especially during the third inflation. Patients had no visible collaterals by angiography and had diameter stenosis less than 70%, in whom hidden collaterals are not commonly found.10-11 Additionally, it is important to emphasise that in our patients, the adaptation to ischaemia was observed in the absence of significant enhancement in distal coronary pressures quantified by the CFI. Consequently, in our study, the values of CFI were somewhat lower than in several previous studies,8-9, 24,30,32-33 and significant signs of ischaemia were present at the first balloon inflation in all patients. This presumably is due to the fact that average stenosis severity was somewhat less than in most other studies and, as a result, collaterals were less developed. At the same time, our patients were symptom-free for several weeks and anti-ischaemic and anti-anginal treatments, including beta-blockers, Ca2+ antagonists, nitrates, as well as other drugs that produce a reduction or induction of indices of preconditioning during PTCA, had been stopped at least 3 days before PTCA.
Recently Argaud et al25 using the SPECT imaging during two consecutive 2.5-min balloon occlusions separated by 5 min reperfusion reported that preconditioning does occur in patients without collateral recruitment. Curiously, they included patients with severe stenosis (mean diameter stenosis greater than 80%), in whom hidden collaterals are commonly found.8-9 The possible explanations for their result are: the recirculation of 99Technetium-sestamibi in the blood pool immediately after balloon deflation, at a time of reactive hyperaemia, could be a confounding factor31 and also, due to the limited spatial resolution, SPECT can only detect collateral perfusion at the myocardial level, averaging out transmural differences.25
The absence of an improvement in ventricular function in patients with no detectable collaterals observed by Sakata et al22 is in accordance with our findings. With inappropriate recruitment of collaterals, an improvement of LV function during repetitive brief periods of ischaemia would be theoretically harmful because of an increase in oxygen requirements.
An increased QTd indicates heterogeneity in the recovery of ventricular excitability, constituting a sensitive and simple arrhythmogenic marker, particularly in ischaemic cardiopathy.13,34-35 In our patients, QTd was significantly greater during the first balloon inflation/deflation than during the second inflation/deflation, and especially during the third inflation/deflation.
Takase et al36 observed that ischaemic preconditioning altered QTd when the treated lesions were located in arteries that irrigated areas of viable myocardium. QTd was not altered when the intervention was performed in vessels that supplied myocardium areas with previous infarction. In our study, we selected patients with no previous infarction and with LV function preserved. Thus, progressive reductions in QTd during repeated balloon inflations indirectly indicate that preconditioning protects against the consequences of electrical instability.
Accordingly, our findings indicate that repeated three 2-min balloon inflations during angioplasty in patients selected on the basis of clinical and angiographic characteristics indicative of a very low likelihood of both significant recruitable collaterals and pre-intervention interference to preconditioning, result in progressive decreases in chest pain, ST-segment shift, and QTd, with no change concerning the left ventricular function, powerfully suggesting the existence of preconditioning in human myocardium.
Study limitations
This study was carried out on a relatively small sample of patients, however, it was performed on a highly selected group of patients and the consistency of findings is promising. Further studies using the same strict protocol are needed to confirm our results on a larger scale. We may have underestimated diameter narrowing due to the known limitations of angiography-QCA due to the fact that the reference segment may have been diseased. In spite of several examples of evidence of absence of collateral protection during balloon occlusion, we cannot totally exclude the possibility of some contributing effect of collateral flow. A more precise assessment of collateral flow, although impractical, would require data from simultaneous measurements of intracoronary pressure and flow velocity, something that was not performed in the present study.

