Abstract
Aims: Patients with cryptogenic embolic events and a patent foramen ovale (PFO) are at risk of paradoxical embolism causing recurrent cerebral events; however, transcatheter PFO closure remains controversial. The aim of this multicentre trial was to demonstrate the feasibility and safety of transcatheter closure of PFO with the HELEX Septal Occluder.
Methods and results: The study enrolled 128 patients (66 female; mean age, 50 years). Mean (±SD) PFO size was 10±3.7 mm; 38 patients also had an atrial septal aneurysm. Device implantation was successful in 127 patients. Device-related events during implantation or follow-up were device embolisation, wire-frame fracture, and retrieval cord breaks (two cases each; no sequelae). Other adverse events included atrial arrhythmia (two patients), migraine, convulsion, and transient ischaemic attack (one case each). There were no recurrent strokes, deaths, perforations, or accumulations of thrombi on the device. Within a mean follow-up period of 21±11 months, complete PFO closure using one device was achieved in 114 patients (90%). Five patients with a moderate to large residual shunt received a second device.
Conclusion: The HELEX Occluder can be used for PFO closure. Device- and procedure-related complications are rare. The closure procedure appears to reduce recurrence rates of stroke and transient ischaemic attack.
Introduction
Up to 40% of ischaemic strokes that occur in young adults have no identifiable cause1 and are therefore considered “cryptogenic”. An association between cryptogenic stroke and paradoxical embolism by means of a venous-to-arterial shunt through a patent foramen ovale (PFO) has been recognised2-4. This type of embolism is observed only rarely on autopsy or imaging studies, so the diagnosis is generally presumptive5. However, a PFO, which is present in approximately 20% to 35% of adults6, has consistently been found to be more prevalent in patients with cryptogenic ischaemic stroke than in either patients with a known cause of stroke or healthy controls2,3,7-9.
Patients with PFO and cryptogenic stroke are at increased risk of recurrent stroke, transient ischaemic attack (TIA), or both, with rates ranging from 1% to 7% per year10,11. Rates may be even higher in patients with additional risk factors, especially an atrial septal aneurysm (ASA)12,13 or a large PFO13. In an effort to prevent recurrent embolic events, patients with paradoxical embolism are generally treated with either medical therapy (antiplatelet or anticoagulant agents), surgical closure of the PFO, or occlusion of the PFO with a device implanted percutaneously through a catheter. No randomised, controlled clinical trials comparing outcomes with these therapies have been reported. However interest in transcatheter PFO closure is increasing. Unlike drug therapy and its side effects14, this technique provides a potentially permanent solution; and unlike surgery, it does not require cardiopulmonary bypass with its attendant morbidity15.
The most widely used transcatheter PFO closure devices are composed of nickel-titanium (nitinol) and polyester (Amplatzer; AGA Medical Corp, Golden Valley, MN) or MP35N (steel) and polyester (Cardioseal; NMT Medical Inc, Boston, MA). Initial results with these and other devices have been promising16-27, although long-term experience is lacking and it has not yet been proved that their use reduces the rate of recurrence of embolic events to levels below those associated with surgical or medical therapy. In 1999 we began to use a double-disc, nitinol-framed, expanded polytetrafluoroethylene (ePTFE) device (HELEX Septal Occluder; W.L. Gore & Associates, Flagstaff, AZ, USA) for this purpose. In studies in dogs28, this device was found to be simple to insert and remove. It also showed resistance to wear and demonstrated long-term biocompatibility, including encouragement of tissue attachment. Small numbers of HELEX devices have been used successfully in several clinical series of PFOs and atrial septal defects20,22,26,29,30. Here, we describe our initial results in the largest series thus far of HELEX Septal Occluder implantations in patients with a PFO (128 patients). Our focus was the safety of the device during insertion and follow-up and its effectiveness in eliminating or reducing right-to-left shunt.
Methods
Patients
Patients were considered for enrolment in this study if they had a recognised PFO and a history of symptoms consistent with an episode of presumed paradoxical embolism and were referred to our institutions between December 1999 and December 2001. Exclusion criteria were active infection at the time the transcatheter procedure was scheduled, pregnancy, thrombus at or near the PFO on transesophageal echocardiography (TEE), an excessively large ASA, an atrial septum thickness of more than 8 mm, and PFO characteristics that would require more than one HELEX Septal Occluder for closure. The last four criteria were assessed during catheter placement in preparation for insertion of a HELEX device.
The HELEX device was used within its CE-mark indication. The patients’ written informed consent was obtained, and all procedures were performed in accordance with the ethics guidelines of the responsible committee at the authors’ institutions and the Declaration of Helsinki.
Device-insertion technique
The HELEX device is composed of a single piece of nitinol wire to which ePTFE is attached along its entire length (Figure 1).
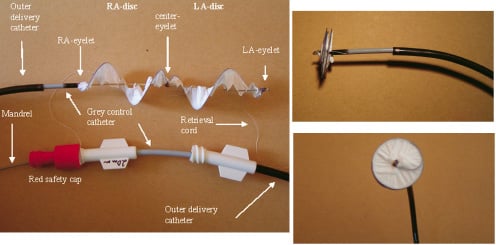
Figure 1. HELEX Septal Occluder. (Left) Device with the delivery system partly stretched out. LA denotes left atrium; RA right atrium. (Top right) Lateral view, showing low profile of the device. (Bottom right) View of LA disc.
At deployment, the super-elastic nitinol wire forms the frames of two opposing discs of equal size that bridge and occlude the PFO. The wire frame is visible on fluoroscopy and has three eyelets that facilitate placement. The occluder is fixed in place by an integral locking mechanism that passes through the centre of the device from the disc in the left atrium to the disc in the right atrium. Available disc sizes range from 15 mm to 35 mm (5-mm increments).
The delivery system for the HELEX device (Figure 1) is a coaxial catheter assembly consisting of a 9 French (Fr) outer delivery catheter, a 6 Fr inner control catheter used to deploy and withdraw the device, and a central mandrel for configuring the device and deploying the locking mechanism. An ePTFE retention suture anchored to the tip of the control catheter loops through the right atrial eyelet to hold the occluder on the catheter as it is exteriorised. The suture allows removal of the device, if necessary, after complete deployment and release from the delivery system.
The HELEX implantation procedure can be performed under fluoroscopic control and with the patient under local anaesthesia though the requirement in some centres for simultaneous TEE necessitates general anaesthesia. The delivery catheter is inserted into the left atrium through the femoral vein. The diameter of the PFO is assessed by using a standard balloon-sizing technique20. A TEE evaluation is conducted to determine whether thrombi or a large ASA is present and to assess atrial septal thickness. Heparin (10,000 U) is administered. In patients remaining eligible for device insertion after the evaluation, incremental advancement of the control catheter and retraction of the mandrel are done to produce constitution of the left atrial disc (indicated by appearance of the centre eyelet). The HELEX system is then gently retracted to ensure apposition of this disc to the atrial septum. The right disc is delivered in a similar manner.
Proper positioning of the device is confirmed fluoroscopically and by ultrasonography. The mandrel is withdrawn into the control catheter to deploy the lock and separate the device from the catheter. The position of the device and leakage status are assessed. If removal of the device is necessary at this point, the retention suture is pulled to unlock the device and withdraw it into the delivery catheter. Otherwise, the retention suture is removed.
Postprocedure regimen
Administration of anticoagulant, antiplatelet, and antibiotic agents after the insertion procedure was done in accordance with protocols used by individual physicians, although all patients received some antiplatelet therapy. Patients were routinely discharged from the hospital the day of or the day after the insertion procedure. Details of each patient’s insertion procedure (PFO size, anaesthesia time, fluoroscopy time, and adverse events) were noted for subsequent data compilation and analysis.
Follow-up study
The purposes of the follow-up protocol were to ascertain the degree and persistence of a right-to-left shunt after insertion of the HELEX device and to record any device-related adverse effects and recurrent embolic events. Therefore, follow-up evaluations consisting of physical examinations and transthoracic echocardiography or TEE studies (or both) were conducted before hospital discharge; at 4 weeks, 6 months, and 1 year after device insertion; and if embolic symptoms developed. Residual right-to-left shunt was classified in a manner similar to that described by Webster et al.7 Thus, we counted the number of bubbles observed on echocardiography in the left atrium within three cardiac cycles from their appearance in the right atrium (ie, the number of bubbles crossing the septum). A finding of no bubbles was assumed to indicate complete disappearance of the shunt; 1 to 5 bubbles, a small/trivial shunt; 6 to 25 bubbles, a moderate shunt; and more than 25 bubbles, a large shunt.
Data analysis
Analyses were conducted on an intent-to-treat basis. Results for continuous variables are presented as mean, standard deviation (SD), minimum, and maximum values. The frequency of occurrence of events are expressed as percentages.
Results
Patients
Between December 1999 and December 2001, a HELEX device was inserted in 128 patients (62 male and 66 female; mean age, 50 years; range, 14-80 years) with a PFO who met the criteria for the study. Thirty-eight of the patients (30%) had an ASA. In 126 patients, the indication for PFO closure was a presumed paradoxical embolism causing a neurologic event (TIA or stroke). Twenty-nine patients had more than one event before PFO closure. One patient had a spinalis anterior syndrome; another had decompression events whilst scuba diving.
Device-deployment experience
We implanted 36 devices of 15 mm, 53 of 20 mm, 28 of 25 mm, 10 of 30 mm, and 1 of 35 mm. The mean device-defect ratio was 2.2. Although a HELEX occluder was inserted successfully in all patients, two device embolisations occurred. In one patient, the device embolised immediately after the procedure and the embolisation was detected during a postprocedure TEE assessment. This patient lacked an anterior rim and had a large atrial septal aneurysm, and a stretched PFO diameter of 17 mm. A 30-mm HELEX device embolised to the aortic bifurcation, where it was removed with a snare, without sequelae. A 19-mm Amplatzer device was then implanted during the same procedure. Use of the HELEX device was therefore considered unsuccessful. In another patient, a 15-mm device embolised within 24 hours after insertion and the embolisation was detected by routine predischarge transthoracic echocardiography. This patient did not have an atrial septal aneurysm, but the stretched PFO diameter was 17.2 mm. The device was snared in the aortic bifurcation, and the PFO was closed with a 25-mm HELEX device.
In 28 patients, the HELEX device that was initially inserted had to be exchanged for another HELEX device one to four times (median, twice per patient) before the permanently implanted device was in place. The reasons for the exchanges are listed in Table 1.
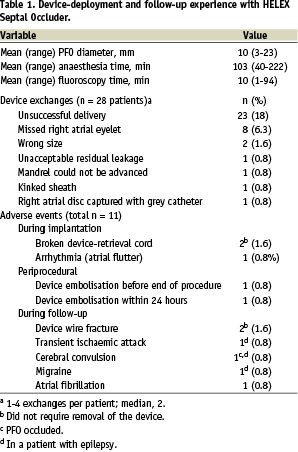
Removal and replacement of the devices did not cause any clinical complications or sequelae. In two patients, the occluder was left in place even though the locking loop did not catch the right atrial eyelet (it was missed), because both discs became well attached to the septum and complete closure of the PFO was achieved. Other data pertaining to the device-insertion procedure, including anaesthesia and fluoroscopy time, are shown in Table 1.
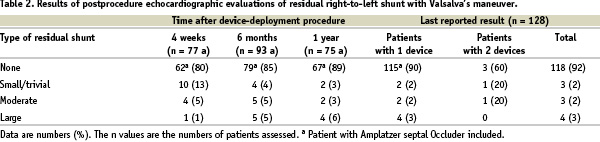
PFO occlusion and follow-up data
A residual shunt assessment with Valsalva´s maneuver was performed immediately after insertion of the HELEX device or before hospital discharge in 124 patients. In 80 patients (65%), total occlusion of the PFO was achieved by the time of hospital discharge. Nineteen patients (15%) had a small/trivial residual shunt, 21 patients (17%) a moderate shunt and 4 patients (3%) a large shunt at discharge.
The mean (±SD) follow-up time was 21±11 months. Results of evaluations of residual shunt at different assessment times are shown in Table 2.
Overall, residual right-to-left shunt resolved completely in 115 of the 128 patients (90%) with one device. A series of TEE evaluation is shown in Figure 2.
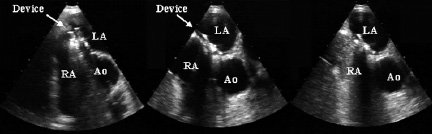
Figure 2. Transesophageal echocardiograms (short axis view) obtained (from left to right) immediately after insertion of a HELEX device, 6 months later, and 6 months after insertion, with bubble contrast visible on the venous side. Ao denotes aorta; LA left atrium and RA right atrium.
In five patients with a persistent moderate to large residual shunt 1 year after device implantation an additional HELEX occluder was implanted. Total PFO occlusion was achieved in three of these patients. The other two retained, respectively, a small residual shunt and a moderate residual shunt.
Adverse events during follow-up are listed in Table 1. One patient in the series had a TIA; however, in this patient the shunt assessment showed complete PFO closure. One patient had newly recognised atrial fibrillation 6 months after device implantation and was treated with an anticoagulant agent. There were no device- or PFO-related deaths, strokes, cardiac perforations, air embolisms, groin hematomas, or instances of accumulation of thrombi on the device.
The patient receiving an Amplatzer Septal Occluder did not have any complication during a follow-up period of 26 months and no residual shunting at any time.
Discussion
In a series of 128 patients with a PFO and presumed paradoxical embolism in whom a HELEX occluder was implanted, we found the device to be safe and effective in achieving PFO closure. Adverse events were rare and without serious sequelae. Total PFO occlusion was achieved in 90% of patients during follow-up. Three of the five patients given a second device also had complete PFO closure.
Table 3 shows patient characteristics, device-insertion information, and outcome variables in large series (> 100 patients) in which various transcatheter devices were used to obtain PFO closure.
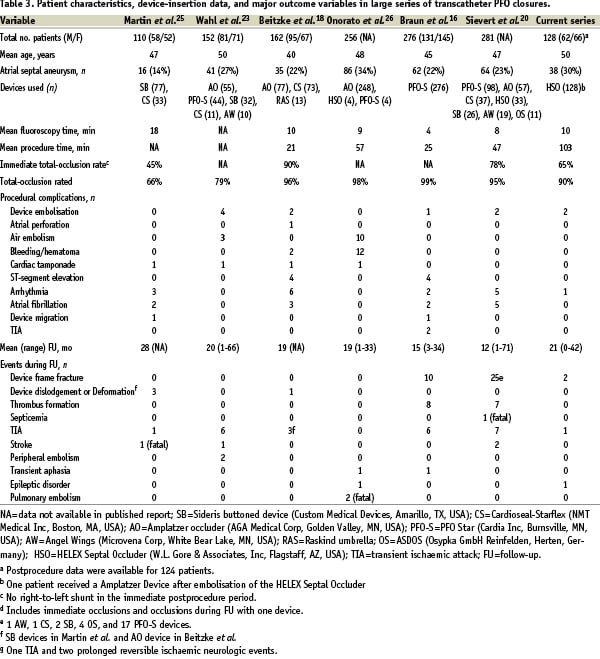
Some of these devices are not available anymore for reasons like perforations with the Angelwings and the ASDOS and residual shunts with the Sideris. In all series, the principal indication for the procedure was presumed paradoxical embolism. The percentage of patients with an atrial septal aneurysm ranged from 14% to 34%. The devices used were implanted successfully in at least 97% of cases in all series listed, including the current series using the HELEX device, in which 99% of our implantations were successful. Mean fluoroscopy time in the series ranged from 4 to 18 minutes; mean procedure time ranged from 21 to 103 minutes. The fluoroscopy and procedure times in our series appear relatively long compared with those in other series. However an assessment of the mean fluoroscopy time for the first 10 patients treated by each interventionalist in our study and the time for subsequent patients showed a decrease from 17 to 7 minutes. This marked reduction in fluoroscopy time is indicative of a learning curve for use of the HELEX device.
Total PFO occlusion rates during follow-up in the series with different devices summarized in Table 3 ranged from 66% to 99%. Thus, our initial results with the HELEX device were comparable to those in large studies in which other occluders were used for PFO closure.
Rates of important periprocedural complications in the seven published series ranged from about 2% (in our series) to about 10%. Device embolisation occurred in five of the seven series, including ours, but the incidence was low. Air embolism and cardiac tamponade were observed in two and four series, respectively, though not in the HELEX series.
Device-related complications during follow-up in the seven clinical series included several wire fractures. These fractures occurred most frequently with the PFO-Star device (Cardia Inc, Burnsville, MN)16,20. Two of the HELEX devices used in the current series had a wire-frame fracture, but neither had to be removed because the PFO remained closed and no fracture-associated adverse events occurred.
In our series, whenever the position of the device or delivery catheter did not appear optimal or a marked residual leak was observed, the entire HELEX system was removed and exchanged for another one. This happened in 28 patients, without any associated adverse effects. Increased experience with deployment of the HELEX occluder and proper disc-size selection enabled the interventionalists in our series to avoid device removal and replacement.
We did find that our original deployment technique resulted in incomplete lock-eyelet capture in eight cases (ie, the right atrial disc was not firmly affixed to the centre eyelet of the device). In these cases, the devices were removed uneventfully by using the delivery catheter retrieval cord or, if the retrieval cord had already been removed, a snare. In two patients, a HELEX occluder with an incomplete lock-eyelet capture was left in place because of a good functional result despite this situation. Later in the series, the cause of incomplete lock-eyelet capture was eliminated by modifying our deployment technique.
Intracardiac echo has been shown to be an alternative to TEE for closure of intraatrial communications26,31. Most patients do tolerate the TEE probe well with little sedation. Especially in those patients who do not tolerate the TEE-probe, intracardiac echo might be chosen to avoid general anesthesia.
Long-term experience with percutaneous transcatheter PFO closure to prevent stroke is still lacking, and randomised studies are required to determine whether it has clear safety and efficacy advantages over medical therapy or surgery. The follow-up data shown in Table 3 provide good evidence of the effectiveness of the technique. Use of the HELEX Septal Occluder offers the possibility of avoiding the morbidity associated with surgery or life-long anticoagulant therapy in patients with a PFO at risk of recurrent embolic events.
Acknowledgements
This research was supported in part by a grant from W.L. Gore & Associates, Inc, Flagstaff, AZ, USA. The authors thank Linda Lamphere and Warren Cutright at W.L. Gore for assistance.
Dr. Peter Ewert, Dr. Fernando A. Maymone-Martins, Dr. Shakeel Qureshi, Prof. Dr. Horst Sievert, and Dr. Neil Wilson are clinical proctors for the Helex device in Europe.

