Abstract
Objectives: We evaluated the long-term clinical and angiographic results of ‘fused-gold’ (NIRFlex Royal) and ‘bare’ (NIRFlex) stainless steel stents in patients undergoing percutaneous coronary intervention (PCI).
Background: Recent studies have shown high clinical and angiographic restenosis rates following the intracoronary implantation of ‘gold-coated’ stainless steel stents. The new ‘fused-gold’ stent, with improved surface characteristics and flexibility, was developed to improve procedural and long-term results, while maintaining enhanced radiopacity.
Methods: A total of 305 patients (358 lesions) with symptomatic native coronary artery disease (CAD) undergoing native vessel PCI were randomised to receive a ‘fused-gold’ (n=147) or ‘bare’ (n=158) stent. Primary endpoint was minimal luminal diameter (MLD) at 6 months angiographic follow-up. Secondary endpoints included technical and procedural success, major adverse cardiac events (MACE), target vessel failure (TVF), angiographic binary restenosis rates, and additional angiographic comparisons.
Results: There were no major differences in the baseline angiographic variables or patient characteristics between the two groups, however there was a trend towards a higher risk in the ‘fused-gold’ stent group. Clinical and angiographic follow-up was 100% and 87% respectively. MLD at 6 months follow-up was smaller in the ‘fused-gold’ stent group compared to the ‘bare’ stent group (1.61±0.65 vs. 1.81±0.60 mm, respectively); Therefore, the null hypothesis of non-inferiority cannot be rejected (p=0.49); equivalency cannot be claimed for the two stent types. The ‘fused-gold’ stents were also associated with a higher angiographic binary and clinical restenosis rates (33 vs. 18%; p=0.002 & 26.9 vs. 20.3%; p<0.001, respectively).
Conclusion: The ‘bare’ NIRflex stent was associated with excellent long-term clinical and angiographic results. Taking into account the equivalence margin, the null hypothesis of non-equivalence between the ‘fused-gold’ NIRflex Royal stent and the ‘bare’ NIRflex stent cannot be rejected (p=0.49), so equivalence cannot be claimed for the two stent types.
Introduction
The majority of intra-coronary stents in clinical use today are constructed from stainless steel. Stainless steel is relatively radiolucent compared to other stent materials, such as tantalum or cobalt-chromium alloy. Radio-opaque stents offer the potential clinical advantage of facilitating more accurate stent positioning within the target lesion during PCI1,2. The radio-opacity of stainless steel stents can be enhanced either by increasing strut thickness or coating stents with a more radio-opaque metal. Increasing strut thickness results in an undesirable decrease in stent flexibility. Gold has been used to enhance stent radio-opacity, as it is biologically inert3-5. Gold-coated stainless steel stents have been shown to be associated with reduced neointimal proliferation compared to other stent coatings in animal studies, and with decreased platelet activation and thrombus formation in vitro3,6-8. However, recent studies have shown an increased rate of restenosis with gold-coated stents9-12. This prospective randomised trial sought to assess the long-term clinical and angiographic results of new ‘fused-gold’ (NIRFlex Royal) and ‘bare’ stainless steel stents (NIRflex) of the same geometric design (both Medinol Ltd., Israel) in symptomatic patients undergoing PCI of native coronary arteries.
Methods
Study population
Patients were eligible if they had symptomatic CAD, with one or more de novo or non-stented restenotic lesions of the native coronary circulation suitable for stent implantation. The visually estimated reference vessel diameter was 2.5-4.0 mm, and lesion length < 30 mm. Exclusion criteria were recent CABG or PCI within 2 weeks, acute myocardial infarction within 3 days, LVEF of < 25%, or patients unable or unwilling to undergo repeat coronary angiography. Angiographic exclusion criteria were excessive vessel tortuosity likely to prevent insertion of an intracoronary stent, unprotected left main disease, ostial or bifurcation lesions, chronic total occlusions, intra-luminal thrombus, in-stent restenosis, or any contraindication to emergency coronary artery bypass surgery. The local ethics committees approved the study protocol. Written, informed consent was obtained from all patients. Patients also gave written, informed consent before undergoing follow-up angiography at 6 months, performed in accordance with institutional guidelines. A subset of patients underwent intravascular ultrasound (IVUS) imaging immediately post-procedure and at follow-up.
Stent implantation
Patients were randomised to receive either a ‘fused-gold’ (NIRFlex Royal) or a ‘bare’ (NIRflex) stainless steel stent of the same geometric design (Medinol Ltd., Tel Aviv, Israel). Randomisation took place after angiographic confirmation of lesion suitability. All patients received combination anti-platelet therapy, consisting of aspirin > 100 mg per day for an indefinite duration and clopidogrel 300 mg pre-procedure and 75 mg once daily for 30 days post-procedure, or ticlopidine 250 mg twice daily 1 day pre- and for 14 days post-procedure. Heparin was administered to achieve an activated clotting time of > 250 seconds. A glycoprotein IIb/IIIa inhibitor was used at the discretion of the operator. Target vessel angiography was performed after the administration of intra-coronary nitroglycerin (100-200 µg) to ensure maximal vasodilatation. Stent length and diameter were determined by visual estimation or quantitative coronary angiography (QCA) with a stent to distal reference vessel ratio of between 1:1 to 1.1:1.0. Direct stenting (without balloon pre-dilatation) was performed at the discretion of the operator. Stents were post-dilated, when necessary, using high-pressure balloon inflations to obtain optimal stent expansion.
QCA / IVUS imaging
QCA was performed at pre- and post-procedure and at 6 months follow-up. The 6 months follow-up angiography was performed in the same technique as above, with same angiographic angles that were taken pre- and post- stent implantation. IVUS was performed at 6 months follow-up using one of two commercially available systems (CVIS/Boston Scientific Corp., San Jose, CA, USA or JOMED, Sweden) with mechanical pullback of the IVUS catheters (3.0 Fr ‘Atlantis’ or 2.9 Fr ‘AVNAR’) at 0.5 mm/sec. IVUS images were recorded on Super-VHS.
Off-line quantitative coronary angiography and IVUS
Off-line QCA and quantitative IVUS were performed by an independent core laboratory (Cardialysis BV, Rotterdam, The Netherlands) according to established methodology13,14. In-stent restenosis at follow-up was classified using the Mehran classification15. IVUS analysis presented in this paper was performed within the stented segment. QCA was performed both within the stented segment and the total analysis segment.
Definition of end points and follow-up
Primary endpoint was in-stent MLD at 6 months angiographic follow-up. Secondary endpoints were technical and procedural success, occurrence of MACE (death, myocardial infarction, and repeat target lesion revascularisation), target vessel failure (TVF), angiographic binary restenosis rates, acute gain and late loss at six months follow-up. IVUS measurements (in-stent neointimal and percentage obstruction volumes) were also reported. Death was defined to include all-cause mortality. Myocardial infarction was defined as new pathological Q waves or by an increase in the creatine kinase (CK), or it’s CK-MB iso-enzyme, of more than or equal to twice the upper limit of normal. CK was determined pre-procedure and at least twice at the following intervals: 6-8, 8-16 and 20-24 hours post-procedure. Angiographic restenosis was defined as > 50% diameter stenosis by QCA within the stented segment. Target vessel revascularisation was defined as a repeat percutaneous or surgical revascularisation involving the original target vessel within 6 months of the index procedure.
Statistics
Power calculations were performed based on the results a previous study using the ‘bare’ NIR (Boston Scientific, San Jose, CA, USA/Medinol, Tel Aviv, Israel) with an expected MLD of 1.88 mm±0.6316. Two group, one-sided t-test (alpha=0.05) will have a 90% power to reject the null hypothesis that the two stents are not equivalent with 171 patients in each group undergoing follow-up angiography. All data expressed as the mean value±SD. The analysis was performed on the actual stent received. Differences in normally distributed continuous variables were tested by the unpaired Student’s t-test. Categorical data were assessed by Fisher’s exact test. A p-value < 0.05 was considered statistically significant.
Results
Patient and procedural characteristics
Three hundred and five patients (358 lesions) undergoing native vessel PCI were randomised to receive a ‘fused-gold’ (n=147) or ‘bare’ (n=158) stent. There were no major differences in the baseline patient characteristics between the two groups, however there was a trend towards a higher risk in the ‘fused-gold’ stent group (Table 1).
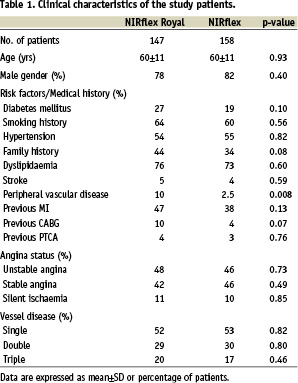
Baseline qualitative angiographic variables were comparable between the two groups (Table 2).
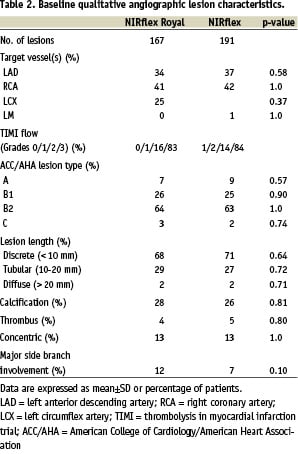
The maximum balloon size and pressure used in the ‘fused-gold’ and ‘bare’ stent groups were similar at 3.3±0.4 vs. 3.3±0.5 mm (p=0.84) and 15.3±4.7 vs. 15.5±3.1 atms (p=0.73) respectively.
Clinical follow-up
All 305 patients were available for one month clinical follow-up. Six month clinical follow-up was available for 304 patients (Table 3).
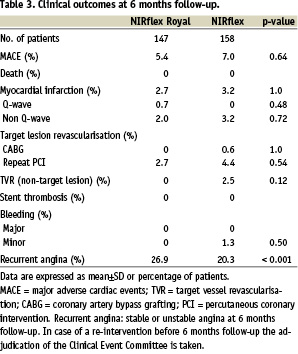
No deaths were observed during the study. Technical success in the ‘bare-stent’ group was 100%, in the ‘fused-gold’ group 96% (p=0.028). Procedural success for both groups was 97 and 94% (p=0.28). A small percentage of patients experienced a myocardial infarction, either peri-operatively or within the first 30 days, in both groups (1.4 vs. 2.5% in the fused-gold’ and ‘bare’ stents respectively: p=0.69). There were no incidences of SAT in either stent at 270 days follow-up. Clinical restenosis was more frequent in the ‘fused-gold’ stent group (Table 3). In the ‘fused-gold’ stent group 83% of patients remained MACE-free at 270 days, compared to 88% in the ‘bare’ stent group (p=0.25). The majority of these events were repeat revascularisation via PCI (14.3 vs. 8.9% respectively: p=0.15). In the patients who underwent repeat PCI, symptomatic or documented ischaemia was documented to be present based on clinical symptoms, prior to repeat angiography, in 74% (n=14/19; no information for 2 additional patients) of those who received ‘fused-gold’ stents, compared to 50% (n=6/12; no information for 2 additional patients) with ‘bare’ stents (p=0.26).
Angiographic analysis
There was a trend towards a larger MLD and a lower percentage diameter stenosis in the ‘fused-gold’ stent immediately post-procedure (Table 4).
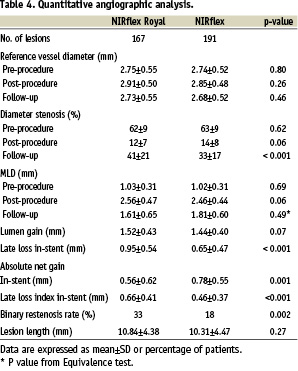
At 6 months 265 patients (311 lesions) underwent follow-up angiography. The ‘fused-gold’ stent was associated with a lower MLD, greater late loss and a higher binary restenosis rate at follow-up compared to the ‘bare’ stent group (Table 4). Taking into account the equivalence margin for MLD at follow-up, the null hypothesis of non-equivalence cannot be rejected (p=0.49), so equivalence cannot be claimed for the two stent types. Patterns of in-stent restenosis were similar with both stents (focal: - 45 vs. 63%, p=0.16; in-stent: - 28 vs. 10%, p=0.09; proliferative: - 21 vs. 23%, p=1.0; & total occlusions: - 6 vs. 3%, p=1.0; ‘fused-gold’ vs. ‘bare’ stents respectively). In the IVUS sub-group, QCA at follow-up suggested no significant differences between the ‘bare’ and ‘fused-gold’ stent group (Table 5).
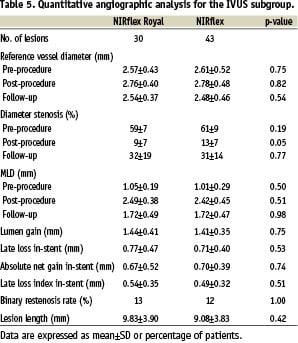
IVUS analysis
Table 6 summarises the quantitative 3-D data at follow-up in the IVUS sub-group.
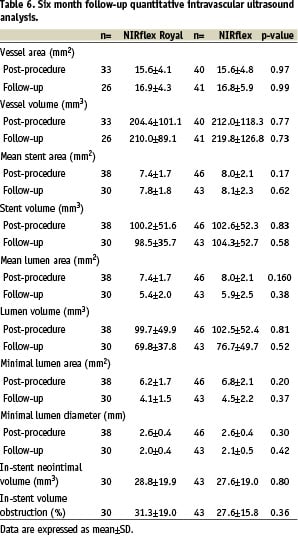
Of the vessels that underwent follow-up IVUS imaging, in-stent lumen, neointimal and percentage obstructive volumes were similar in the two stents.
Discussion
This prospective randomised study did not demonstrate equivalence in the long-term results of the ‘fused-gold’ NIRflex Royal and ‘bare’ NIRflex stainless steel stents. The results of the ‘bare’ NIRflex stent were excellent, with low angiographic binary restenosis rate and low late loss. The NIRflex Royal stent was associated with greater angiographic late loss, reduced MLD and higher angiographic and clinical restenosis rates at follow-up compared with the NIRflex stent. However, MACE and target vessel revascularisation rates at 6 months were similar, with no incidences of SAT with either stent.
The IVUS sub-study was included to address concerns over the possible methodological difficulties with current QCA algorithms in the evaluation of the radio-opaque NIRflex Royal stent, as has been previously recognised with other highly radio-opaque stents17,18. Such difficulties may, in part, explain the observed trend towards a higher post-procedure MLD with the NIRflex Royal stent seen in this study despite similar baseline lesion characteristics and stent implantation technique.
IVUS also allowed a blind analysis of the radio-opaque and radiolucent stents. In this sub-study, in contrast to the clinical and angiographic restenosis results and in agreement with the similar MACE, IVUS demonstrated similar in-stent neointimal and percentage obstruction volumes in the two stents. However, the QCA results at follow-up (MLD, percentage diameter stenosis and late loss) in this subgroup were also similar, indicating that these patients were either not typical of the entire study population, or that they were subject to IVUS-guided therapy (Figure 1).
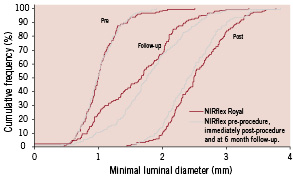
Figure 1. Cumulative frequency distribution curve of minimal lumen diameter.
The baseline angiographic characteristics of the patients enrolled in this study were remarkably similar to those enrolled in recent drug-eluting stents trials, with a reference vessel diameter of around 2.75 mm and total lesion length of < 15 mm. The 6 month angiographic results of the ‘bare’ NIRflex stent in this study are intermediate between those reported with paclitaxel- and sirolimus-eluting stents and other ‘bare’ metal stents, although this requires confirmation in a ‘head-to-head’ randomised trial16,19-28.
Despite the previous evidence that gold is bio-compatible, the non-equivalent clinical and angiographic results of the NIRflex Royal in this study are similar to those of previous studies using gold-coated stents9-12. It appears that post-plating thermal processing of the gold coating on the NIRflex Royal stent, in an attempt to negate the adverse tissue response, is insufficient when deployed in human native coronary arteries, although incompatibility with current QCA algorithms cannot be excluded3.
Study limitations
The radio-opacity of the ‘fused-gold’ NIRflex Royal prevented a double-blinded comparison of the two stents. Although IVUS imaging did allow blinded analysis, only a small number of patients were entered into this subgroup. Follow-up IVUS measurements only included vessels which underwent repeat IVUS imaging. The clinical symptomatic status at follow-up was not collaborated with non-invasive testing in the majority of patients.

