Abstract
Pre-clinical as well as early phase human studies have demonstrated the ability for cell therapy to augment perfusion and increase myocardial contractility. In general, the effects are modest and often similar despite differences in the study design, cell number or type. Accordingly, number of issues should be addressed before stem cell therapy could become the standard clinical practice. They are related to selection of the optimal cell type, standardization of the cell processing and release criteria. Other issues include timing of the cells injections and cell homing and retention. Further research is needed to understand the mechanisms underlying observed functional and beneficial effects including optimization of myocardial biological effects. A close interaction between translational and clinical research is needed to address these conceptual or procedural issues. Consequently, the efficacy and safety profile needs to be established in the well-designed clinical trials.
Introduction
Stem cell therapy is emerging as a potential therapeutic option for cell death-related heart diseases1. Pre-clinical as well as early phase human studies have demonstrated the ability for cell therapy to augment perfusion and increase myocardial performance. In addition, recent intermediate size randomised trials suggested the potential of bone marrow stem cell (BMC) to augment left ventricular (LV) recovery after recent myocardial infarction. In general, the effects are modest and often similar despite differences in the study design, cell number or type. Therefore a number of issues should be addressed before stem cell therapy could become standard clinical practice (Table 1).

Clinical trials
There are several strategies for using bone marrow cells as a novel treatment in ischaemic heart disease. Most of the current studies used mononuclear bone marrow stem cells (Table 2). This approach is based on the assumption that no potentially beneficial cell type is omitted and that the functional recovery depends on the equilibrium between all cell sub-populations present in the mononuclear fraction. In addition, using this approach, autologous stem cells can be easily harvested and processed via bone marrow aspiration and require little or no ex vivo expansion. However, while early observational studies2-4 suggested a significant effect on left ventricular (LV) function, the results of randomised trials are controversial. The REPAIR-AMI trial reported a modest, but a significant improvement in global and regional LV function at ventricular angiography which persisted at 12 months5. On the other hand, the BOOST trial showed a similar improvement in left ventricular-ejection fraction (LVEF) at six months as compared to controls6; however, the difference in LVEF disappeared at 18 months due to recovery of the LV function in controls7. In addition, Janssens et al reported a significant effect of BMC on infarct size as compared to placebo that did not translate into the superior effect on the functional recovery8. The recently published ASTAMI trial failed to find a significant improvement in the LVEF as assessed from either cardiac magnetic resonance imaging, single photon emission tomography or echocardiography9. The reasons for lack of consistent effects of BMC on left ventricular function in randomised trials remain unclear and may be related to differences in the study design, patient’s selection, timing of cells injection or methods used to assess functional response. Furthermore also differences in cell number and bone marrow processing among this various studies may account for this inconsistency. For instance, in the ASTAMI trial9, mononuclear bone marrow cells (BMNCs) were not injected immediately after the processing, but cells were injected after overnight culture. It should also be noted that in the REPAIR-AMI trial3, patients with EF lower than 50% had a higher benefit of cell administration with a better clinical course and less adverse events as compared to the cell treated group of patients with EF higher than 50%.
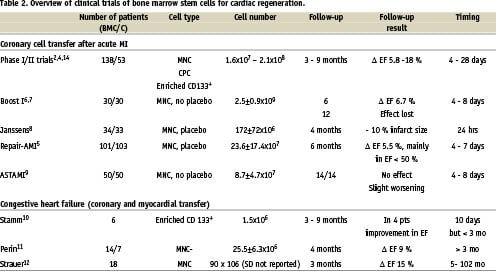
The potential of the bone marrow cells in the setting of congestive heart failure was studied only in the small, non-randomised clinical trials10-12. Therefore a number of ongoing randomised trials should seek to establish the benefit of the current generation of the bone marrow-derived cell products in this setting.
Issues with the cell type
Despite the encouraging findings of beneficial functional effects following intracoronary injection of bone marrow mononuclear stem cells in patients with acute myocardial infarction, the function of many cell types in the mononuclear fraction is unknown and progenitors with known function such as mesenchymal cells or CD34+ and CD133+ cells consist only a very low portion of the entire fraction2-4. In addition, multiple progenitors are likely to compete for engraftment during the trans-endothelial passage, thereby impeding the homing of cells with well known effects. In this regard, recent findings suggest almost a seven fold higher homing capacity of enriched haematopoetic cells as compared to unfractionated mononuclear cells after intracoronary injections in patients with myocardial infarction13. It is also interesting to note that in the Top-Care studies, functional effects upon cardiac recovery were similar after transfer of circulating endothelial progenitors cells or bone marrow-derived mononuclear stem cells suggesting the presence of a common cell type mediating the functional response3. Finally, it remains unclear whether inflammatory progenitors, mostly present in the mononuclear fraction, are beneficial or cloud the functional improvement. It seems possible that these effects will depend on the time of cell injection in relation to the initial myocardial injury (see below). Taken together, this advocates for the studies that would address the contribution of well defined cell types to cardiac recovery.
There remain other cell-related issues16-18. Experimental studies indicate that progenitor cells with high haematopoetic and angiogenic activity are critical mediators of functional effects and show angiogenic and myogenic potential in response to growth factor treatment. In addition, c-kit+, lin- or CD34+ cells mediate cardiac repair by inducing angiogenesis, inhibiting apoptosis and promoting myocyte recovery in experimental myocardial infarction14,15. In humans, pro-angiogenic effects of CD133+ cells were suggested by improved myocardial perfusion after injections into the chronically infarcted myocardium at the time of the bypass surgery11. In a pilot non-randomised controlled trial, our group recently demonstrated an improvement in global and regional LV function which was paralleled by a better perfusion and higher metabolic activity in the infarcted area following IC administration of a selected population of CD133+ cells14. Prospective double blind randomised trials are initiated to further explore this approach. Alternatively, mesenchymal cells represent a pluripotent cell population with angiogenic effects15.
Apart from which cells are being used, there remain also some safety issues16-18. Although allogeneic cell compounds must be prepared under stringent laboratory conditions, autologous cell preparation is not standardised, especially when cells are to be injected soon after harvesting. Moreover the angiogenic potential of autologous cell compounds is not routinely evaluated before their administration and the only relatively controlled parameter is the number of cells. The percentage of sub-population of cells also varies, imposing further complexity on cell dosing. These hurdles complicate the ability to assess the dose-and-effect relationship, a factor of paramount importance in the safety and efficacy of the therapeutic compounds.
Timing and homing issue
The course of the infarction healing as well as the presence of putative homing signals within the damaged myocardium appear to favour the cell engraftment during the trans-endothelial passage in the early days after reperfusion. On the other hand, the adverse inflammatory environment with high oxidative stress may be deleterious if cells were to be administered too early after reperfusion. Current knowledge of the inflammation over time is summarised in Figure 1. Stem cell mobilisation is seen peaking very early, and decreases typically between seven and 14 days after MI. Cytokines modulating the adhesion are peaking around day 7 and remain elevated up to four weeks, migratory cytokines are only very transiently increased. Initial ischaemia and reperfusion injury is characterised by the rapid rise of reactive oxygen radicals followed by the early rise of pleiotropic inflammatory cytokines within hours after MI peaking between day three and seven to 10. Finally, proteins regulating the matrix formation and healing begin to rise at one week after the injury.
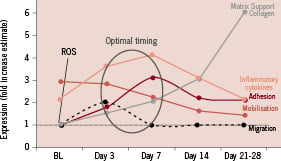
Figure 1. Time course of factors related to stem cell homing, modified from Bartunek et al19.
Given the time course of all these factors, one would assume that the optimal time window for IC stem cell therapy should be around day 3-7, when the balance between putative and detrimental factors is most favourable for cell homing and survival19. This assumption is supported by recent double-blind placebo-controlled trials. Janssens et al, reported no effect on the global LV function after coronary transfer of BMNCs within 24 hours after acute myocardial infarction (AMI)8. On the other hand, sub-analysis of the REPAIR-AMI study5 indicated that beneficial effects of IC transfer were observed only if the cells were delivered later than four days after successful reperfusion. It is also interesting to note that in the non-randomised trials, beneficial effects were seen even when cells were injected beyond one week, leaving the boundaries of the time window in the later stages undefined2,4,14. In this regard, the group of Zeiher and Strauer noticed small, but significant effects on the functional recovery even after intracoronary delivery in patients with old myocardial infarction at least if the myocardial infarction was less than three years old12,3. It is obvious that a better understanding of the temporal dynamics of homing signals and BMC retention in the infarcted myocardium may provide critical clues into the timing of the stem cell therapy.
Human studies evaluating the distribution and engraftment rates using this intracoronary application are limited. Hofmann et al studied the immediate bio-distribution of BMNCs after intracoronary injections in patients with recent acute myocardial infarction using [18F]-fluoro-2-deoxy-D-glucose (FDG) labelling at positron emission tomography13. They showed a markedly higher homing of enriched CD34+ cells in the area of culprit vessel as compared to non-selected BMNCs. Recently, Penicka et al studied the one-day kinetics of myocardial engraftment of autologous BMNCs after intracoronary injection using 99mTc-HMPAO nuclear imaging in patients with acute and chronic anterior myocardial infarction. They demonstrated that myocardial retention substantially decreased in the first 20 hours after coronary transfer from ~ 5 to ~ 1%. In addition, in patients with chronic myocardial infarction, myocardial activity disappeared in all patients20.
Mechanistic issues
Proposed mechanisms for the beneficial effects of stem cell therapy include cardiomyocyte and/or endothelial differentiation, cell fusion and/or paracrine effects. The issue remains controversial but several studies suggested that bone marrow stem cells could co-localise with non-myocardial cells such as fibroblasts21 or could lead to presence of calcifications18. These observations are so far anecdotal, and could be explained by either methodological issues like dye release from dead cells in the area of fibrosis; or use of unfractionated bone marrow, which include several precursor populations. Nevertheless, they are consistent with the basic postulate of stem cell therapy that stem cells may follow milieu-dependent differentiation. Conceptually, damaged adult myocardium being devoid of key embryonic growth factors may not be able to recapitulate the necessary environment to stimulate myocyte growth or regeneration. This poses a challenge to the basic postulate of the cell therapy and call for the close scrutiny of biological myocardial effects of transplanted cells using appropriate imaging tracking or molecular tagging. Accordingly, the search for novel strategies to coax cells toward a cardiac lineage, prior to transplantation to follow the “controlled” path of differentiation or regeneration should be investigated (see below).
Framework of the optimisation
Framework of future studies, we learned that a framework of future studies should be devised in order to fully exploit the potential of the cell based therapies. This framework for optimisation should cover at least four aspects.
First, the outcome of the therapy should be better tailored to the given patient. Future studies need to confirm observations from the REPAIR-AMI trial that patients with lower ejection fraction could be indeed the main benefactors of the cell therapy5. For the future translation of the therapy, it is critical to demonstrate benefits in patients at higher risk. Hypothesis driven studies should specifically address whether patients with moderate and severe LV dysfunction, long ischaemic times or presence of microvascular dysfunction could benefit from the stem cell delivery. It is intriguing to note that patients with low ejection fraction and large myocardial injury show low systemic levels of circulating progenitors early after infarction. Though these observations do not explain whether such patients are truly poor mobilisers, or whether low levels of circulating bone marrow cells reflect higher myocardial uptake in case of greater myocardial injury, it should be addressed whether and how naturally occurring bone marrow mobilisation and its interplay with injured myocardium relates to effects of exogenously administered stem cells.
The second aspect to consider in the future framework is the cell product and it’s processing. Besides the above mentioned issues surrounding the cell choice, it is becoming apparent that cell processing and its methodology may matter for the stem cell function and outcome. The Frankfurt group recently demonstrated that Ficoll based separation resulted in: higher absolute cell numbers including the number of bone marrow stem cells; higher in vitro and in vivo functional capacity, including induction of neovascularisation in the hind/limb ischaemia model as compared to lymphoprep separation (Scientific Sessions AHA 2006). The Ficoll based protocol was superior for freshly isolated as well as for cultured mononuclear cells. These differences in cell number and function may account for absence of the therapeutic effect in the ASTAMI trial as compared to REPAIR-AMI study. Thus, for any cell type or separation method, extensive in vitro standardisation and release criteria including precise description of cell number and in vitro and in vivo functional capacity should be established prior initiation of the pre-clinical or clinical study. In addition, bone marrow stem cell function may be altered in patients with cardiovascular risk factors, and a number of approaches are being explored to improve their function such as cell engineering or use of adjunctive pharmacotherapy including statins or hormones22.
Thirdly, manipulations are being explored that improve cell homing. Factors and mechanisms accounting for transient cell retention are possibly related to the adhesive status of the micro-circulatory compartment or to the functional features of BMNCs. This has to be addressed in future clinical and experimental studies using serological or tissue cytokine profiling. In addition, further studies are needed to investigate whether pharmacological modulation of microcirculation at the time of cells injection could enhance cell homing and abolish its transient nature. For instance, BMC homing may be probably denied by the “no-reflow” phenomenon which might be improved by the co-administration of a vasodilator23. On the other hand, novel approaches for optimisation of the delivery need to be further explored. An alternative to exogenous delivery of autologous stem cells is to identify the mechanisms by which stem cells are recruited to the heart and try to augment mobilisation of stem cells, particularly in the setting of acute infarction24. Concerning this aspect, the cytokine stromal-derived factor (SDF)-1 and its cognate receptor CXCR4 have emerged as important players in the homing and mobilisation process. Activation of hypoxia-induced transcription factors induces ischaemic tissues to produce SDF-1 that attracts circulating CXCR4 positive stem cells. Modifications of this pathway may be promising for enhancement of the cell homing and retention25.
In addition, as demonstrated by Hofman et al13, enrichment of the cell product for a particular cell type may result in superior engraftment as compared to mononuclear cells, raising a more simple and immediate alternative to test in the specific hypothesis driven study. The evaluation of cell homing and retention is critical for our understanding of mechanisms and efficacy of the cell therapy and requires development of new labelling techniques that would allow precise tracking of the cells as regards their viability, migration and fate/differentiation. Given the concerns related to the effect of current tracers on the cell function and differentiation capacity26, the use of new modalities such as perfulocarbon nanoparticles could be important.
Finally, the controversy around the myocardial effects led to the suggestion that alternative strategies such as coaxing the cells toward the cardiac lineage or addressing critical molecular pathways of cardiac differentiation should be investigated as an approach to increase the functionality of the cells27. This was demonstrated in several experimental studies using genetic modifications. Mesenchymal stem cells pre-conditioned in a hypoxic environment upregulate Akt expression28 and stem cells induced to over-express HGF increase capillary density, reduce collagen content, and improve cardiac function in the rat infarct model29. On the other hand, extensive knowledge has been accumulating on factors and signalling pathways providing the basis for strategies based on the biological modifications of stem cells30.
Conclusion
Despite the promising results of initial observations and randomised trials, cell-based treatment will only leave the grey zone when definite answers are found for the aforementioned questions. The progress should follow the well designed path (Figure 2) and co-ordination, such as that which was proposed in the recent report of the ESC task force on stem cell therapy31. We should follow the path of randomised, preferably placebo controlled trials, for those cell types/technologies that passed the test of randomised trials with surrogate end-points. New hypothesis driven studies should address specific issues such as homing, cell delivery or cell type choice. This will require continuous bench and bedside feedback. Finally, continuous safety scrutiny should remain the primary goal in these studies.
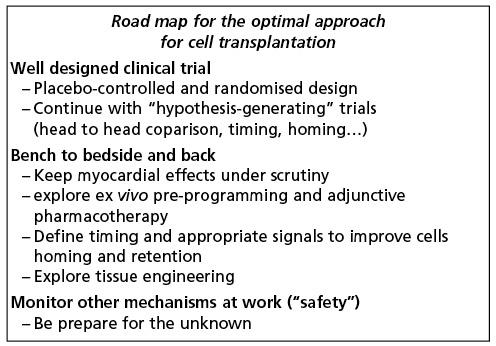
Figure 2. Road map towards the optimisation of the current therapeutic approaches.

