Abstract
Objectives: Recently, concerns were raised about the relative long-term safety and efficacy of drug-eluting stents (DES) in saphenous vein bypass grafts (SVG). Our objective was to assess the 4-year relative safety and efficacy of the unrestricted use of drug-eluting stents (DES) as compared to bare metal stents (BMS) in saphenous vein bypass grafts (SVG).
Methods: Between April 16, 2002 and December 2005 a total of 122 consecutive patients were treated with either sirolimus- or paclitaxel-eluting stents for saphenous vein graft disease. These patients were compared with 128 consecutive patients treated with BMS in the immediate preceding period (January 1, 2000 to April 2002).
Results: At 4-years the cumulative survival rate in the DES group was 77.5% versus 73.0% in the BMS group (adjusted HR 1.09; 95% CI 0.63-1.90, Logrank p=0.65). The cumulative survival free of major adverse cardiac events (MACE: death, myocardial infarction and target vessel revascularisation) was 61.5% vs. 46.8% in the DES and BMS groups respectively (adjusted HR 0.77, 95% CI; 0.51-1.16) due to a higher event free survival of clinically driven target vessel revascularisation in the DES group as compared to the BMS group (81.6% vs. 69.0%; adjusted HR 0.53; 95% CI 0.27-1.05).
Conclusions: In the present study, the use of DES for SVG PCI was associated a similar safety profile and there was a trend towards lower rates of TVR and MACE at four years as compared to BMS.
Introduction
Saphenous vein grafts are the commonest conduit in coronary artery bypass graft surgery (CABG).1 However, the lifespan of saphenous vein grafts (SVG) proved to be limited – at 10 years, 50% of such grafts contain at least one significant stenosis with a total occlusion rate of up to 40%.2,3
Currently the use of drug eluting stents (DES) for off-label indications is frequent (up to 60 % in our centre) and PCI has surpassed CABG as the treatment of first choice for treating coronary artery bypass graft disease.4,5 Still, event-free survival after stent implantation remains low due to restenosis at the lesion site.6-8 The use of DES in SVG lesions has led to a decrease in restenosis and the need for repeat revascularisation at one year as compared to bare metal stents (BMS).9-11
Currently there is still scarce evidence about the long-term safety and efficacy of DES when used in coronary artery bypass grafts. The recently published 32-months follow-up of the Delayed Reduction of Restenosis In Saphenous Vein Grafts With Cypher Sirolimus-Eluting Stent (RRISC) trial showed a catch-up in the repeat revascularisation rates in patients treated with sirolimus-eluting stents (SES).12 Moreover, the authors reported a significant increase in late mortality in patients treated with SES as compared to those treated with bare metal stents (BMS).12
The current study was performed to assess the long-term outcome of a consecutive series of patients treated with BMS, sirolimus- or paclitaxel-eluting stents (SES and PES respectively) for lesions in venous bypass grafts.
Methods
Patient selection
Between January 1, 2000 and December 31, 2005 a total of 387 percutaneous interventions were performed in our institution using BMS, SES or PES in coronary bypass-graft lesions (arterial or venous bypass grafts) (Figure 1).
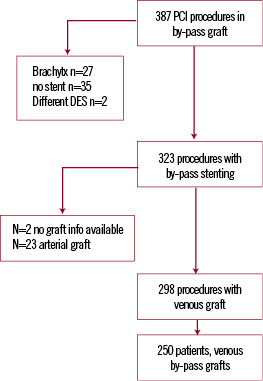
Figure 1. Inclusion flow chart of study population.
A total of 62 procedures were excluded due to treatment restricted to balloon angioplasty (n=35) or the use of (previous) brachytherapy (n=27). Two patients received a Symbiot™ Covered Stent and were also excluded. Out of 323 procedures selected, 298 involved the treatment of saphenous vein grafts and in 25 procedures arterial grafts were treated. From January 2000 until April 16th 2002, 144 PCI procedures in a venous bypass-graft were performed using exclusively BMS, from April 16, until December, 2005, 154 procedures were performed using either sirolimus-eluting stents (Cypher®, Cordis Corp., Johnson & Johnson, Warren, NJ, USA) or using paclitaxel-eluting stents (TAXUS™ Express2™ or Liberté™, Boston Scientific, Natick, MA, USA).
Patients initially enrolled in one of the sequential cohorts (BMS or DES) were maintained for analytical purposes throughout the follow-up period in their original cohort, even if a repeat intervention was performed using a different type of stent at a later stage. Finally, 250 patients fulfilled these criteria.
This study was approved by the local ethics committee and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Procedural and baseline definitions
All procedures were performed following previously defined current standard procedural guidelines.13 The use of distal embolisation protection devices and periprocedural glycoprotein IIb/IIIa inhibitors were left to the operator’s discretion. Finally, the use of distal protection devices was low (4.7% in the BMS group vs. 1.6% in the DES group; p=0.28).
Patients were prescribed aspirin plus clopidogrel 75 mg/day (after a loading dose of 300 mg) before or during baseline coronary interventions. Patients treated with bare metal stents received at least one month of clopidogrel (median, three months, IQR: 2-6 months). Patients treated with DES received at least three months of clopidogrel (median, six months, IQR: 6-6 months). All patients were advised to remain on aspirin indefinitely.
Hypertension was defined as a blood pressure > 140 systolic or > 90 mm Hg diastolic or based on the current use of antihypertensive treatment. Dyslipidaemia was classified as a total serum cholesterol level of > 6.2 mmol/l or the use of lipid lowering drugs. Diabetes was defined as treatment with either oral hypoglycaemic agent, insulin or through diet. Complete procedural success was defined by the achievement of <50% diameter stenosis (visual assessment) and Thrombolysis in Myocardial Infarction (TIMI) grade flow 3 in all lesions intended to treat. Clinical success was defined as procedural success without death or re-infarction during the index hospitalisation.
Endpoint definitions and clinical follow-up
Our primary endpoint was MACE (major adverse cardiac events; defined as a composite of all-cause death, myocardial infarction [MI] and target vessel revascularisation [TVR]) at 4-years. Secondary endpoints included the itemised outcomes all-cause death, MI and TVR at 4-years. MI was defined as creatinine kinase-MB enzyme elevation > 3 times the upper limit of normal. TVR was defined as a clinically driven (presence of clinical symptoms and/or signs of ischaemia) repeat revascularisation procedure (either percutaneous or surgical) of the index graft. Stent thrombosis (ST) was defined as angiographically defined thrombosis with TIMI grade 0 or 1 flow or the presence of a flow limiting thrombus, accompanied by acute symptoms, resembling the ARC definite criteria.14,15
Survival status was obtained from municipal registries. Cause of death was acquired via the Central Bureau of Statistics, The Hague, The Netherlands and classified according to the international Classification of Diseases and Related Health Problems, 10th revision (ICD-10).16 Questionnaires inquiring about patients current health status, and medication use were subsequently sent to all living patients. Events (MI, TVR) that occurred outside our institution were verified by contacting the peripheral hospital. Finally, follow-up was available for 98.4% of the BMS patients and 95.9% of the DES patients.
Statistical analysis
Summary statistics for all continuous variables are presented as medians together with the interquartile range (IQR). Categorical data are summarised as frequencies and percentages. Continuous variables were compared using the Mann-Whitney U test. Categorical variables were tested for significance using the Chi-square or Fisher’s exact test. Survival and event-free survival analysis were presented using Kaplan-Meier survival curves and tested for difference using the log-rank test. Cox proportional hazards regression models were used to control for differences between groups and independent predictors of outcome. First, all baseline, clinical and procedural variables were put in a univariate cox proportional hazards regression model for the different endpoints. Second, all significant predictors of outcome (p<0.1) were forced into a second model along with stent type (BMS or DES) and tested for significance. Final results are reported as adjusted Hazard ratios (HR) with their respective 95% confidence intervals (CI). All statistical tests were two-tailed. A value of p < 0.05 (unless reported otherwise) was used for all tests to indicate statistical significance. All statistical analyses were performed using SPSS version 12 (SPSS Inc., Chicago, Illinois).
Results
Baseline and procedural characteristics are presented in Tables 1 and 2 respectively.
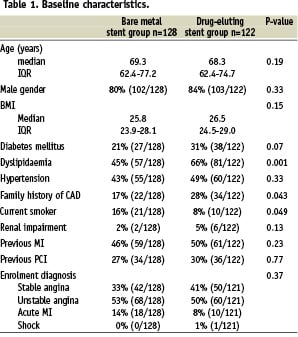
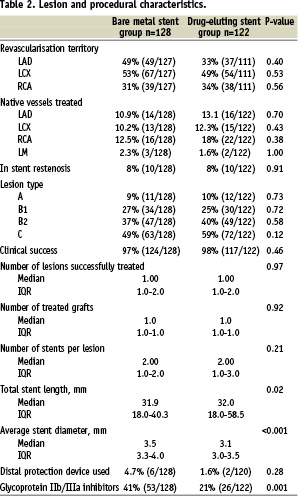
Baseline characteristics were similar between the two groups, except for a significantly higher incidence of family history of coronary artery disease and dyslipidaemia in the DES group as compared to the BMS group. Procedural characteristics differed in terms of a smaller average stent diameter and a longer total stented length in the DES group. The use of glycoprotein IIb/IIIa inhibitors decreased over time, from 41% in the BMS group to 21% in the DES group, p=0.001). At 4-years, the cumulative survival free of MACE was 61.5% versus 46.8% in the DES and BMS groups respectively (adjusted HR 0.77, 95% CI; 0.51-1.16). [Figure 2, Table 3]
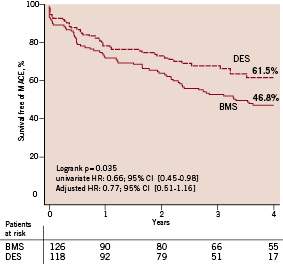
Figure 2. Kaplan Meier event free survival of major adverse cardiac events (MACE, the primary combined endpoints of all-cause mortality, myocardial infarction, and clinically driven target vessel revascularisation). DES stands for drug-eluting stent, BMS for bare metal stent.
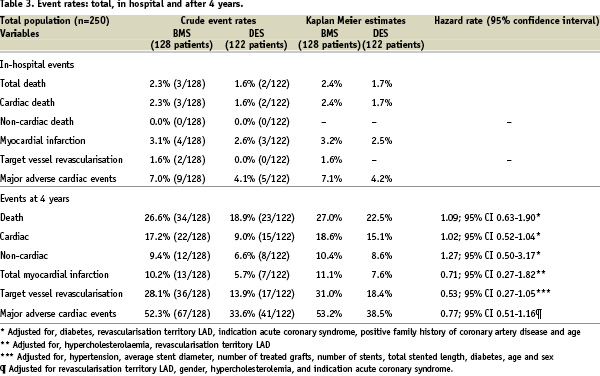
A total of 57 patients died (23 in the DES group and 34 in the BMS group). The cause of death was cardiac in 15/23 (65.2%) in the DES patients and 22/34 (64.7%) in the BMS patients. the cumulative survival rate in the DES group was 77.5% versus 73.0% in the BMS group (p=0.65). When adjusting for independent predictors the HR for death in the DES group was 1.09; 95% CI 0.63-1.90). [Figure 3, Table 3]
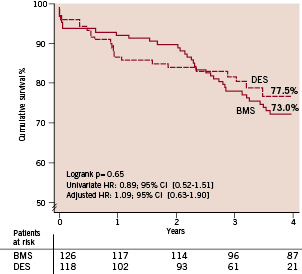
Figure 3. Kaplan Meier event free survival. DES stands for drug-eluting stent, BMS for bare metal stent, TVR for target vessel revascularisation, MI for myocardial infarction and MACE for major adverse cardiac events.
The cumulative event free survival for the combined endpoint death/MI was 70.6 % in the DES group vs. 65.8% in the BMS group (adjusted HR 1.11 95% CI; 0.68-1.81).
Cumulative survival free of clinically driven TVR was higher in the DES group as compared to the BMS group (81.6% vs. 69.0% respectively; adjusted HR 0.53; 95% CI 0.27 - 1.05). [Figure 4, Table 3]
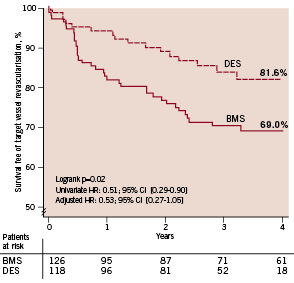
Figure 4. Kaplan Meier event free survival of clinically driven target vessel revascularisation. DES stands for drug-eluting stent, BMS for bare metal stent, TVR for target vessel revascularisation.
A total of five (4.0%) patients treated with BMS suffered from stent thrombosis occurring at a median of 176 days (IQR 134-731) versus only 1 (0.8%) in the DES group occurring at 606 days.
Discussion
The present study demonstrates that the use of DES in SVG remains safe and effective as compared to BMS up to four years of follow-up, illustrated by similar survival rates and a trend towards significantly lower rates of TVR in patients treated with DES. At four years, the use of DES tended to result in lower MACE rates, mainly caused by lower repeat revascularisation rates in the first year in patients treated with DES – even though definite conclusions cannot be drawn, this is a risk reduction comparable to that observed in both the general PCI population and in SVG stenting.9,11,17-19
The 32-months results of the randomised Delayed RRISC trial showed a catch-up in the repeat revascularisation rates in patients treated with SES along with a significant increase in late-mortality as compared to BMS.12 Unfortunately, the sample size of the latter study was calculated based on in-stent late loss, which explains the sample size of only 75 patients and the highly selected patient population. Patients presenting with MI, with impaired renal function, distal graft lesions, chronic total occlusions were excluded, along with those presenting with aorto-ostial or calcified lesions making the results difficult to apply in real-world clinical practice. Yet, it was difficult to question these findings given the lack of long-term data regarding the safety of DES in SVG and the presence of previously raised concerns about a catch-up in the reintervention rates in diabetics and patients presenting with ST-segment elevation myocardial infarction treated with DES.20,21 Thus far, individual patient level data meta-analyses of the pivotal randomised Cypher and TAXUS trials were not able to address this issue given the lack of high-risk patients and larger (network) meta-analyses simply precluded subgroup analyses due to the lack of the individual patient data.17,18,22,23 To date, large-scale registries have not yet reported on the long-term outcome in this specific patient subset.24,25
The present study included a total of 250 real world consecutive patients treated for SVG disease of which the vast majority did not undergo routine angiographic follow-up. At four years, both all-cause and cardiac survival were identical between the BMS and DES group and there was no sign of a catch-up in TVR rates following DES use. The importance of detailed analyses of high-risk subgroups can be demonstrated by several recent studies suggesting that DES perform best in high-risk patients, like those presenting with small vessels, long-lesions, diabetes and SVG.24,26 Thus far, the overall benefit of DES has been widely adopted, but concerns regarding their long-term safety17,25,27 prompted investigators to further scrutinise their data for cost-effectiveness and heterogeneity of the treatment effect. While thus far, the safety concerns seem to be unfounded, a proper patient selection might become of crucial importance given the unfavourable cost-effectiveness profile of the DES.26
The steep drop in the TVR-free survival in the BMS group forced us to scrutinise the indications leading to the re-interventions occurring between 100 and 260 days. Only clinically driven cases of TVR were taken account into the present analysis. Out of the 14 TVR procedures occurring at six months in the BMS group, only two were due to angiographic follow-up and were not counted in the present analysis. Out of the remaining 12 patients who underwent a repeat intervention within this time frame, eight presented with unstable angina, three with stent thrombosis and one patient presented with stable angina and had a positive stress test.
Although patients treated with DES received clopidogrel for a longer period of time, the prescribed duration of clopidogrel did not seem to impact on any of the endpoints, even when adjusting for independent predictors.
The present study has several limitations. Firstly, although the DES and BMS groups in the present study were reasonably well matched in terms of baseline and procedural characteristics, it remains uncertain whether the use of extensive regression analyses was able to fully correct for the dissimilarities between the groups. Nevertheless, the overall risk profile was greater in the DES group. Large-scale randomised trials are needed to prove the long-term benefit advantage of DES over BMS in SVG. Secondly, the drug-eluting stent cohort contained both patients treated with SES and PES. However, no heterogeneity in the treatment effect was found regarding the use of either SES or PES.
Conclusions
In the present real world patient cohort, the use of DES for SVG lesions appeared safe and effective after 4-years of clinical follow-up. At 4-years, the use of DES tended to results in lower MACE rates as compared to BMS, due to similar survival rates and a trend towards lower rates of TVR.

