Abstract
Aims: To study the 5 month performance of a balloon-expandable percutaneous heart valve (PHV) in the systemic circulation of animals.
Methods and results: Moderate-to-severe (≥ grade 3) aortic insufficiency was created in 14 juvenile sheep using a bioptome device. A 23mm PHV was implanted distal to the left subclavian artery (1.78±0.18 cm2 area, 27±2 mmHg closing gradient). Valve performance was followed angiographically (baseline, 21 weeks) and echocardiographically (baseline, 6, 14, 21 weeks). Animals were euthanized at 21 weeks or at the first sign of valve dysfunction. Four animals died of acute procedural complications (aortic perforation, mitral valve damage, hemothorax, and sheath dissection of brachio-cephalic artery). One animal with a rotated PHV (secondary to aortic dissection) was euthanized at 6 weeks; 3 animals were euthanized at weeks 6 and 14 because of decreased leaflet mobility (normal PHV at autopsy, decreased mobility related to halothane-induced hypotension). Six animals with normal PHV function (1.68±0.12 cm2 area, 23±4mmHg closing gradient) were euthanized at 21 weeks; pathologic examination revealed no significant PHV abnormalities. Native aortic valve insufficiency was grade 2-4 at 21 weeks.
Conclusion: PHV function remains satisfactory to 21 weeks in the descending aorta. This animal model is valid for chronic study of PHV in the systemic circulation.
Introduction
With the first human implantations in the pulmonary conduits of children1 and the stenotic aortic valves of elderly patients2, percutaneous valve replacement emerged as a revolutionary technology to treat valvular heart disease. In the course of the development of these methods, a number of animal experiments were conducted to develop implantation techniques and study the behavior of these valves in a biologic system. In a subset of animals, we sought to assess the long-term safety, durability, and performance of an original percutaneous heart valve (Percutaneous ValveTechnologies, Inc - Edwards Lifesciences, Irvine, California, USA) in the systemic circulation. This valve (Figure 1), made of equine pericardium sutured in a balloon expandable stent, was initially conceived for the treatment of degenerative, calcific aortic stenosis3.
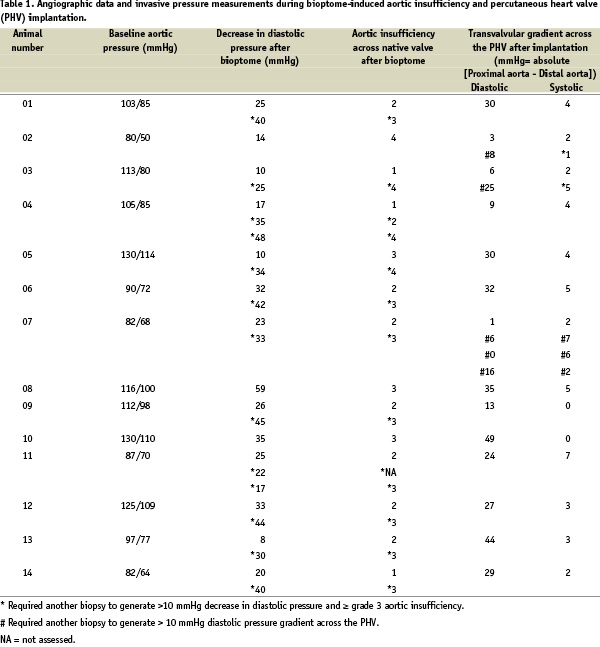
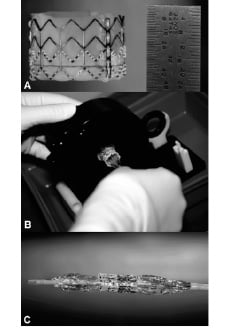
Figure 1. A: Equine pericardial valve sutured to a stainless steel stent that is 14 mm in height. B: Percutaneous heart valve (PHV) is carefully collapsed over a balloon catheter using a specifically designed crimping device. C: PHV mounted on balloon catheter is compatible with introduction through a 24 Fr sheath.
In the healthy animal model, however, percutaneous heart valve (PHV) implantation within the native aortic valve can easily result in valve migration or death because of the lack of calcium, the elasticity of the aortic root, and the anatomic configuration of the coronary orifices and the mitral valve4. Valve implantation in the heterotopic position or descending aorta, would thus be ideal, but consistently leads to early failure due to the lack of a transprosthetic diastolic pressure gradient needed for valve closure4. In 1952 before the advent of extra corporeal circulation, Hufnagel5 surgically implanted mechanical valves into the descending aorta of patients with chronic aortic insufficiency. This technique was able to block the majority of the regurgitant volume and provide lasting amelioration of symptoms in patients for many years6. We thus sought to reproduce, through a percutaneous approach, the Hufnagel technique and create an original animal model of chronic aortic insufficiency in which the durability of the PHV can be studied in the systemic circulation. Below, we describe our 21 week experience with implantation of the PHV in the proximal descending aorta of juvenile sheep with moderate to severe (grade 3) aortic regurgitation.
Methods
Population and preparation of the animals
Animal experimentation was conducted in 14 healthy juvenile sheep (age 8-11 months, weight 36-52 kg) at the BIOMATECH facility in Lyon, France. The study was performed in accordance with the requirements of the FDA “Good Laboratory Practice” and the European regulations for animal experimentation7. Animals underwent induction anesthesia with intravenous thiopental-pentobarbital; maintenance anesthesia consisted of inhaled halothane (1-4%) and nitrous oxide. Transesophageal echocardiography (TEE) was performed to measure the aortic arch diameter. Only animals with a descending aorta diameter of 18-20 mm were accepted for this protocol. The right femoral artery and brachiocephalic trunk were cut down and cannulated with a 7 Fr introducer. Heparin was administered at a dose of 50 IU/kg intravenously and anticoagulation levels were maintained at 2-3 times control level. Aspirin (350 mg) was also given intravenously at the beginning of the procedure. A 6 Fr pigtail catheter was introduced from the femoral artery to the level of the descending aorta to measure baseline pressure and perform aortic angiography.
Creation of controlled aortic insufficiency
A 7 Fr bioptome, typically used for right ventricular biopsy (Cordis, Johnson & Johnson, Miami, Florida, USA), was advanced from the brachiocephalic trunk to the level of the aortic valve. The bioptome was used to extract small fragments of the aortic valve leaflets (Figure 2A). Repeated contrast injections in the ascending aorta and diastolic aortic pressure measurements were used to assess the degree of aortic insufficiency and to guide the direction of the bioptome (Figure 2B).
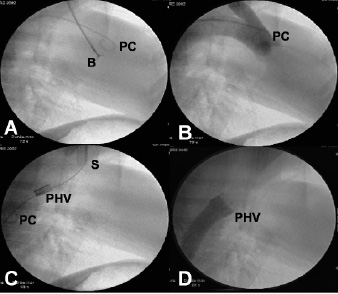
Figure 2. A: Using the pigtail catheter (PC) as a guide, a bioptome (B) is advanced to the level of the aortic valve to remove part of the leaflets and induce aortic insufficiency. B: Supra-aortic angiography through a pigtail catheter (PC) is used to assess the subsequent degree of aortic insufficiency and well visualize the aortic valve. C: The brachio-cephalic artery is cut down and cannulated with a 24 Fr sheath (S) through which the percutaneous heart valve (PHV) is placed distal to the left subclavian artery. Pigtail catheter (PC) in the descending aorta is used to perform angiography and guide placement of the PHV. D: Descending aorta angiography shows a competent percutaneous heart valve (PHV) that prevents filling of the ascending aorta with contrast.
Moderate to severe insufficiency (≥ grade 3) was defined by complete diastolic filling of the left ventricle during supra-aortic angiography and a decrease of diastolic pressure > 10 mmHg compared to baseline values. If the regurgitation was not sufficient after the first biopsy, the procedure was repeated.
PHV implantation
A right Judkins catheter was used to introduce a 260 cm, 0.035-inch stiff guide wire into the descending aorta from the brachiocephalic trunk. The 7 Fr introducer and Judkins catheter were subsequently replaced by a 24 Fr sheath (COOK, Bjaeverskov, DK). Before the PHV could be inserted through the larger sheath, it was carefully collapsed onto a 22 mm Z-MED II balloon (NuMed Canada Inc, Cornwall, Ontario, Canada) using a specially designed crimper. The balloon-valve assembly could then be advanced over the guide wire into the descending aorta, distal to the origin of the left subclavian artery (Figure 2C). Once well positioned, the balloon was rapidly inflated to fully expand the PHV. The balloon was subsequently deflated and removed simultaneously with the guide wire.
Native and PHV valve evaluation
A right Judkins catheter was advanced from the brachiocephalic trunk into the ascending aorta, and a pigtail catheter was advanced from the femoral artery into the descending aorta, distal to the PHV. Simultaneous pressure measurements were used to measure the systolic and diastolic transvalvular gradients. If the diastolic transvalvular gradient was < 10 mm Hg, the bioptome was re-advanced from the brachiocephalic trunk, and biopsies of the native aortic valve were performed until a sufficient gradient was made across the PHV. Angiography in the ascending and descending aorta was performed to confirm placement of the PHV, and assess the degree of regurgitation of both native valve and PHV (Figure 2D). TEE was performed at the end of the procedure to confirm leaflet mobility of the PHV, planimeter the PHV opening area, and assess the degree of insufficiency of the native valve and PHV.
Follow-up
Animals were transferred to a hosting facility where they were observed daily. The sheep were maintained on subcutaneous enoxaparin 40 mg daily for one month and oral aspirin 160 mg daily for the duration of the study. Routine haematological and bio-chemical blood evaluation was performed 1, 2, 3, 4, 5, 12, 16 and 21 weeks after implantation. Measurement of body weight and evaluation with TEE were done at 6, 14 and 21 weeks. Animals showing signs of severe debility or valve dysfunction were euthanized for ethical reasons. All sheep that completed the study (21 weeks) had a repeat angiographic evaluation and were then euthanized. Explanted valves underwent gross and histologic examination at a core pathology laboratory; organs, such as the heart, aorta, spleen, brain, liver and kidneys, underwent only gross examination.
Results
Acute phase
In all 14 animals, moderate to severe aortic insufficiency (≥ grade 3 by angiographic assessment) of the native valve was successfully created with the bioptome device (Table 1). One to 3 biopsies of the native leaflets was required to generate this amount of regurgitation and decrease the diastolic blood pressure by 36±12 mmHg. Following disruption of the native aortic valve, a 23 mm PHV was successfully implanted in the proximal descending aorta of all sheep, with a final valve area of 1.78±0.18 cm2. In three cases (Table 1), the native valve had to be re-biopsied because of an insufficient PHV transvalvular diastolic pressure gradient (< 10 mmHg). Sheep 2 was not re-biopsied a third time because of subsequent low systemic pressure (systolic 50 mmHg, diastolic 32 mmHg). Final diastolic and systolic pressure gradients across the PHV were 27±12 mmHg and 3±2 mmHg respectively. By echocardiography (Table 2), PHV insufficiency (central) occurred in only 3 animals (2 with mild insufficiency, 1 with moderate insufficiency). In one case (sheep 14), moderate regurgitation was associated with a dissection of the descending aorta and extension to the paravalvular area. Native valve regurgitation after implantation was between grade 2 and 4 by TEE assessment.
Four sheep did not have a TEE and died within 24 hours of the procedure. Immediate death occurred after perforation of the aortic arch and biopsy of the mitral valve with consequent severe mitral regurgitation in case 4 and 7, respectively. Sheep 9 died a few hours after the procedure of a large hemothorax secondary to rib fracture during transport pre-procedure and heparin-induced bleeding intra-procedure. Sheep 2 was found dead the following morning secondary to a traumatic aortic dissection from the brachiocephalic sheath, haematoma between the aorta and the PHV, and significant pulmonary congestion. Death was procedure related and not device related in all cases.
Follow-up
At 6 weeks (Table 2), all 10 surviving animals were in good clinical health without laboratory abnormalities, change in eating habits or weight. Seven animals had a PHV with normal leaflet mobility and grade 1 to 2 regurgitation (central). The native aortic valve in these animals had grade 2 to 3 insufficiency (sheep 12 not well visualized by TEE). The remaining three animals were euthanized because of a non-functioning leaflet (one leaflet in the semi-open position in sheep 10 and 11) or a valve that had rotated perpendicular to the aorta with three leaflets in the open position (sheep 14 with known aortic dissection). At autopsy, the valves in sheep 10 and 11 were without any gross abnormalities that could explain this leaflet immobility. The valve in sheep 14 had turned 90 degrees on its axis and the leaflets were in the open position. A large aortic dissection filled with thrombus was observed distal to the valve.
At 14 weeks, 7 animals continued to thrive without clinical signs of valve dysfunction. In 5 of these animals, the PHV had normal leaflet mobility and grade 1 to 2 insufficiency (central). Sheep 13 had one cusp that appeared non-mobile in the closed position, and sheep 6 had three non-mobile cusps in the open position and associated grade 4 regurgitation. The latter animal was euthanized, despite the lack of symptoms, and autopsy revealed no gross calcifications, thrombosis or commissural fusion that could explain this finding.
At 21 weeks follow-up, the 6 remaining sheep continued to do well clinically. Leaflet mobility was normal in sheep 3 and 5. Leaflet mobility initially appeared decreased during echocardiography in sheep 1, 8, 12 and 13, but returned to normal when the dosage of halothane was decreased. Final PHV area in the six sheep was 1.68±0.12 cm2 and central insufficiency was grade 1 by TEE (grade 0 by angiography). Aortic regurgitation across the native valve was grade 1 to 2 by TEE and 2 to 4 by angiography (Table 3).
Mean diastolic gradient was 23±4 mmHg and systolic gradient was ≤ 1 mmHg across the PHV.
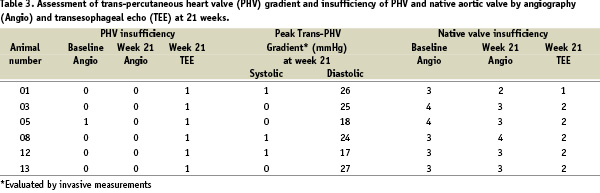
At gross autopsy, the PHV showed only minor abnormalities: limited thrombus formation on one leaflet (sheep 8). Histological examination of the PHV revealed small and focal calcification of one leaflet (sheep 1), mild degradation and angiogenesis of the leaflets (sheep 13), and moderate inflammation of the leaflets (sheep 3, 6, 10 and 11). Inflammatory cells consisted of macrophages and occasional giant cells; in sheep 6, inflammatory cells also consisted of lymphocytes. These findings were similar to those seen in surgical bioprosthetic valves implanted in sheep models8-10. All PHV’s, except one, were completely incorporated in the native aortic wall by well-organized neointimal growth (Figure 3); the PHV implanted with aortic dissection and subsequent rotation (sheep 14) was only partially embedded into the aortic wall with little neointimal coverage of the stent struts.
Figure 3. A: High power electron microscopy depicts stent struts (SS) and filaments (F) of suture material completely endothelialized. B. High power electron microscopy depicts valve material from the base of the prosthetic leaflets well-incorporated into the wall of the aorta.
The native aortic valves had significant bioptome-induced lesions (Figure 4).
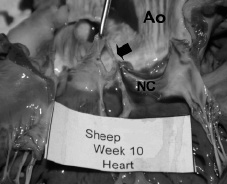
Figure 4. Gross specimen showing a hole in an aortic leaflet (arrow) at 10 weeks in sheep 14 as a result of bioptome-induced injury. (Ao = aorta, NC = non-coronary leaflet of the aortic valve)
Target organs were without gross abnormality except sheep 13 with lymphadenopathy, and sheep 11, 12 and 14 with white spots on the kidney suggestive of an ischemic lesion. Body weight as well as haematological and bio-chemical blood examination remained within normal limits for all animals.
Discussion
The field of percutaneous intervention has expanded to involve implantation of PHV for the treatment of aortic valve disease, the most common valvular abnormality in adults. Currently, there is a need to obtain data concerning the long-term performance of any PHV developed for implantation in the systemic circulation. For studying the 5 month performance of a balloon-expandable PHV, we developed an animal model of chronic aortic insufficiency using an original bioptome technique, and implanted the PHV distal to the left subclavian artery. Except in animals with an initial procedure-related complication, PHV function remained satisfactory up to 21 weeks in the descending aortic position.
Implantation of a PHV into the native aortic valve would be an ideal method to study the chronic performance of these devices. Unfortunately, this type of study is currently impossible in animals because of several, anatomical limitations4. Currently, there is no animal model with calcific stenosis of the native aortic valve which could anchor the PHV, and thus avoid device embolization. Furthermore, the distance between the coronary arteries and valve annulus in sheep is only 4-5 mm, which precludes orthotopic implantation without the risk of coronary obstruction. And though other animal models have a larger distance between the coronary arteries and the aortic annulus (pig model), the distensibility of the proximal aorta and the lack of fluoroscopic markers makes precise placement very difficult. Therefore, our group had attempted to implant the PHV into the descending aorta of juvenile sheep (the animal used in previously established models of surgically implanted bioprosthetic valves)8-10, realizing afterwards that the valve leaflets remain in the open position because of an absence of a closing gradient. Subsequently, PHV implantation in the descending aorta became coupled with the creation of aortic insufficiency4,11,12. In these earliest experiments, aortic insufficiency was created by perforating the aortic leaflet with a transseptal needle, placing a guide wire, and inflating a balloon catheter to disrupt the native valve4. The resulting regurgitation, however, was either too severe or too mild to study the long term function of the bioprosthesis or its protective effects on valvular insufficiency. Animals with severe acute aortic regurgitation did not survive longer than 24 hours. Animals with mild to moderate aortic regurgitation did not have valvular insufficiency at 3 month follow-up (smaller injury to aortic valve healed by fibrosis) and had failure of the percutaneous bioprosthesis (valve remained in the open position)4. Using the bioptome method, we could create a “controlled” injury to the aortic valve which resulted in moderate to severe aortic regurgitation that was well tolerated acutely and remained stable or decreased by one grade over the duration of the study. Procedural death was never related to the aortic regurgitation, but the inadvertent biopsy of the mitral valve, perforation of the aorta, dissection of the aorta and rib fracture during transport.
Though the conditions created were sufficient to report the mid term function of the PHV in 9 animals and the chronic function (5 months) in 6, there is some evidence that these study results may have been negatively affected by the use of our maintenance anesthesia. In 4 of the animals (sheep 1, 8, 12, and 13) at 21 weeks, we observed that higher concentrations of halothane affected the mobility of the PHV leaflets. Halothane, known to decrease systemic blood pressure13, could have contributed to the decrease in the native valve regurgitation and the resultant transprosthetic closing gradient. Unfortunately, this relation was confirmed only at the end of the study and a low closing gradient might explain why 3 animals, prematurely euthanized for leaflet immobility (sheep 6, 10 and 11), had a normal appearing prosthesis on pathologic examination. This relation may also explain the differences in the degree of aortic regurgitation seen throughout the study in the same animal.
Percutaneous implantation of a valve into the heterotopic position after creation of aortic regurgitation is technically simple and useful, not only for studying the long term function of a percutaneous bioprosthesis in the systemic circulation (a requirement by the FDA before the onset of clinical studies on humans), but also to study the protective effects of a competent valve in a model of valvular insufficiency. The earliest ball valves used to treat aortic regurgitation in humans were sutured into the descending aorta distal to the left subclavian artery. These valves prevented as much as 75% of the regurgitant volume14, and patients lived ≥ 10 years without symptoms of insufficiency6. With the advent of bypass machines and oxygenators, mechanical valves and later bioprosthetic valves were surgically implanted into the orthotopic position. Recently, there is renewed interest in reviving the Hufnagel technique for patients with high operative risk, particularly those with previous aortic valve replacement15. In this group of patients, the procedure could be performed percutaneously in patients. Pathologic examination in animals shows the stent frame appropriately endothelializes in the descending aorta and changes in the leaflets (limited calcific deposits, collagen degeneration and inflammation that do not cause valve dysfunction) are similar to those found in surgical bioprosthetic valves implanted in juvenile sheep8-10. In Europe, the performance and durability of the PHV in humans has already shown excellent initial results in the systemic circulation, more than 24 months since implantation into the calcific valves of patients with inoperable aortic stenosis. In addition to the clinical and animal experience, in vitro fatigue studies show that the performance of this equine pericardium valve may last more than 5 years in vivo without degeneration. Further follow-up will show the durability of the equine tissue valve as compared to established bovine and porcine valves.
The initial chronic data from PHV implantation in the systemic circulation is encouraging. This data was generated using an original animal model which should become increasingly important for durability testing of other PHV devices in the future. Implantation of a PHV in the descending aorta may also have a potential role in humans with inoperable aortic insufficiency.
Acknowledgements
Funding was provided by Percutaneous Valve Technologies Inc. - Edwards Lifesciences, the company that designed the percutaneous valve used in these experiments.
Appendix
Pathology Core Laboratory: Renu Virmani, MD, Armed Forces Institute of Pathology, Department of Cardiovascular Pathology, Washington, DC, USA.

