Abstract
Background: The composition of atherosclerotic coronary plaque is a major determinant of future clinical events. Spectral analysis of IVUS radiofrequency data has demonstrated potential to provide detailed quantitative information on plaque composition. We prospectively assessed plaque composition in matched coronary segments at a six-month interval and sought to explore correlations between temporal changes in plaque composition and circulating biomarkers.
Methods and results: Twenty coronary segments (mean baseline length, 29.9±14.1mm) with non-significant angiographic (<50 % diameter) stenosis, in non-culprit vessels of patients (n=20) referred for percutaneous intervention, were studied at baseline and six-month follow-up. Spectral analysis of IVUS radiofrequency data, obtained with a 30 MHz catheter in segments matched on predefined anatomic landmarks, was performed with IVUS-Virtual Histology™ software. After 6 months, an overall decrease in the level of different biomarkers of instability was present. There were no significant changes in absolute values (mm2) of plaque components; calcium (0.036±0.05 vs. 0.033±0.04), fibrous (2.68±1.5 vs. 2.90±1.5), fibrolipidic (0.77±0.4 vs. 0.73±0.4), and lipid core (0.42±0.4 vs. 0.52±0.5), between baseline and six-months. Nor did any conventional IVUS variable (lumen, vessel, or plaque cross-sectional area, or percent area stenosis). Change in lipid core area (r=0.51, p=0.024), fibrous area (r=0.49, p=0.033) and calcium area (r=0.63, p=0.004), were significantly correlated with change in Lp-PLA2 activity.
Conclusions: Routine medical care does not result in significant overall changes in IVUS-derived plaque size or composition over a 6-month period. This study provides indication of quantifiable boundaries beyond which modifications in tissue composition might be interpreted as statistically significant.
Introduction
Despite significant advances in diagnosis and therapy, coronary atherosclerosis remains a major cause of death in developed countries1. Pathological studies have related specific coronary plaque characteristics to fatal ischemic events but conventional imaging techniques cannot reliably identify them prospectively. Coronary angiography is the standard invasive technique to evaluate the presence and extent of coronary atherosclerosis. However, due to the phenomenon of positive vessel remodelling advanced atherosclerotic disease is often present despite only minimal lumen encroachment on angiography2-4. Most future atherothrombotic events will occur at the site of these non-obstructive plaques5. The histological composition of atherosclerotic plaque has been linked to the fate of the plaque as described by Davies et al., who showed that plaques with ≥ 40 % of lipid core harbor a higher risk of undergoing rupture and subsequent thrombosis6. Thus far, although different lipid-lowering strategies have demonstrated a clear clinical benefit, changes in local plaque burden are modest, ranging from no changes to slower progression or at most halting progression of coronary atheroma7-10. Accordingly, a beneficial change in composition might be present and yet remain unnoticed. This hypothesis is supported by experimental, and other in vivo observations9,11.
Plaque characterization through visual interpretation of gray scale IVUS is imprecise, specially when assessing heterogeneous, lipid-rich plaques12. In contrast, spectral analysis of IVUS radiofrequency data has demonstrated potential to provide detailed quantitative information on plaque composition and has been validated in studies of explanted human coronary segments13.
Circulating biomarker levels have been shown to predict clinical events in seemingly healthy subjects and in large cohorts of patients with coronary disease14. The combination of novel imaging techniques and the assessment of circulating biomarkers could have a potential role in refining the risk stratification in patients and eventually aid further development of novel drug therapies.
This pilot study evaluated for the first time, human coronary atherosclerotic plaque composition at two timepoints six months apart with ultrasound radiofrequency data analysis. In addition, we explored the correlation between different plaque components and circulating biomarker levels.
Methods
Patients
The Integrated Biomarker and Imaging Study (IBIS) was a prospective, single center, non-controlled observational study. In this study 84 patients underwent repeated invasive and non-invasive imaging (angiography, IVUS, palpography, multislice computed tomography) at baseline and at 6-month follow-up in the same matched Region Of Interest (ROI). The ROI was determined using identifiable anatomic landmarks (side branch or the ostium of the vessel). Concomitantly, multiple biomarkers were assessed. IVUS-Virtual Histology™ (IVUS-VH) only became available late in the enrollment period and the 20 patients undergoing serial IVUS-VH constituted the present study population.
In the IBIS trial, patients were eligible if they were referred for percutaneous coronary intervention (PCI) and had a non-significant (< 50 %) lesion, within a ROI in a non-intervened vessel that could be safely interrogated with IVUS. Major relevant exclusion criteria were renal dysfunction (creatinine > 2 mg/dl), life expectancy less than one year, or factors that made follow-up difficult. The Medical Ethics Committee of the Erasmus Medical Center approved the study protocol and all patients gave written informed consent. All imaging techniques were analyzed independently. All patients underwent repeat IVUS-VH imaging at 6 month follow-up, in the same matched ROI as illustrated in Figure 1.
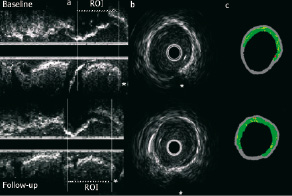
Figure 1. (a) The use of identifiable side-branches as anatomic landmarks ensures that the same region of interest (ROI) is analysed at baseline (above) and follow-up (below). (b) Cross-sectional areas, at the two timepoints, can also be matched using side-branches (star) and other identifiable anatomical landmarks. (c) Virtual Histology™ reconstruction of b, depicting essentially unchanged plaque composition.
IVUS-VH acquisition and analysis
Details regarding the validation of the technique, on explanted human coronary segments, have previously been reported13,15,16. Briefly, IVUS-VH uses spectral analysis of IVUS radiofrequency data to construct tissue maps that classify plaque into four major components. In preliminary in vitro studies, four histological plaque components (fibrous, fibrolipid, lipid core and calcium) were correlated with a specific spectrum of the radiofrequency signal13. These different plaque components were assigned color codes. Calcified, fibrous, fibrolipidic and lipid core regions were labeled white, green, greenish-yellow and red respectively.
IVUS-VH data were acquired, during a continuous pullback (0.5 mm per second) with a commercially available mechanical sector scanner (Ultracross™, 30 MHz catheter, Boston Scientific, Santa Clara, CA, USA), by a dedicated IVUS-VH console (Volcano Therapeutics, Rancho Cordova, CA, USA). The IVUS VH data were stored on a CD-ROM and sent to the imaging core lab for offline analysis (Cardialysis, Rotterdam, the Netherlands). IVUS B-mode images were reconstructed from the RF data by custom software (IVUSLab, Volcano Therapeutics, Rancho Cordova, CA, USA). Subsequently, semi-automatic contour detection of both the lumen and the media-adventitia interface was performed. To account for catheter-to-catheter variability the acquired RF data was normalized using a technique known as “Blind Deconvolution”. Blind deconvolution is an iterative algorithm that deconvolves the catheter transfer function from the backscatter, thus enabling automated data normalization17,18. Compositional and geometrical data were expressed as mean cross sectional areas (CSA, mm2). Plaque area was defined as Vesselarea - Lumenarea and percent area stenosis was defined as [(Vesselarea - Lumenarea)/Vesselarea] X 100. For describing geometrical data plaque was defined as plaque plus media whereas for plaque compositional data the media was not included.
Biomarkers
Blood for biomarker analysis was centrifuged within 30 minutes and stored at –70°C. Serum C-reactive protein (Diagnostic Systems Laboratories), plasma interleukin 6 and tumor necrosis factor-α (R&D Systems), were measured in the Human Biomarker Center (GlaxoSmithKline, PA) with use of protocols provided by the manufacturer. Lipoprotein-associated phospholipase A2 activity assay measures the proportional release of aqueous 3H acetate resulting from the enzymatic cleavage of the 3H acetyl-platelet activating factor substrate (100 µM). N-terminal pro brain natriuretic peptide was measured with use of a two-site electrochemiluminescent assay. The limits of quantification were 0.0048 mg/L for C-reactive protein, 0.057 pg/mL for interleukin-6, 3.92 nmol/min/mL for lipoprotein phospholipase
A2 activity, 10 pg/ml for N-terminal pro brain natriuretic peptide, 0.88 pg/mL for tumor necrosis factor-α, 0.062 ng/mL for sCD40L and 0.13 ng/mL for active MMP-9. Since Lp-PLA2 is known to be associated with LDL in plasma, we also measured LDL cholesterol19,20.
Statistical analysis
Discrete variables are presented as counts and percentages. Continuous variables are presented as means ± standard deviations (SD). Differences in means among groups were analyzed by a two-tailed sample t-test. A P value of less than 0.05 was considered to indicate statistical significance.
Bland Altman analysis plots were used to assess changes between baseline and follow-up21. The limits of agreement were determined by the mean difference between both techniques ± 2 SD.
Comparisons between quantitative outcomes were performed with use of scatterplots and linear regression analysis (regression coefficient). Biomarkers were not normally distributed except for lipoprotein-associated phospholipase A2 activity. Thus, where appropriate, analyses were performed after natural logarithmic transformation. We looked for correlations between imaging endpoints and circulating biomarkers and calculated the univariate Pearson correlation coefficients. Statistical analyses were performed with use of SAS Version 8 (SAS Institute, Cary, NC).
Results
Baseline patient (n=20) characteristics are presented in Table 1.
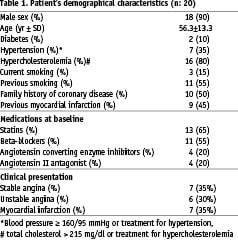
The population was relatively young, with a low (10%) prevalence of diabetes; and 65% of the patients were receiving statin therapy at baseline. The mean values of the conventional IVUS variables, at baseline and follow-up, are presented in Table 2.
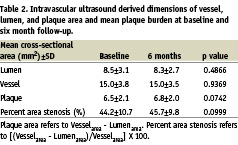
Of note, the length of region of interest was the same during serial studies (29.9±14.1 mm vs. 29.3±13.8 mm, NS). After 6 months, there were no significant changes in LDL-cholesterol (Δ 0.07±0.3 nMol/L, p=0.36) and HDL-cholesterol (Δ –0.09±0.2 nMol/L, p= 0.09) levels.
Although there were no significant changes in mean vessel or lumen cross-sectional areas, there were non-significant trends towards an increase in absolute plaque area and percent area obstruction (Table 2).
Plaque characterization using IVUS-VH
Absolute values for major plaque components (calcium, fibrous, fibrolipidic and lipid core), at baseline and follow-up, are presented in Table 3.
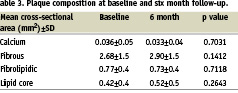
Overall, there were no significant changes over time. These results are illustrated in graphic form in Figure 2.
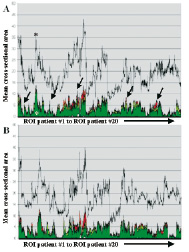
Figure 2. Plaque composition in all 20 patients is plotted sequentially on the x axis (A. Baseline, B. Follow-up). The y axis shows the total percent area stenosis (colored peaks), the composition (calcium: white, fibrous: green, fibrolipidic: greenish-yellow and lipid core: red) and the external elastic membrane (EEM, gray line). After 6 months, no major overall changes in coronary atherosclerosis regarding plaque areas and compositional components are evident in all available cross-sections.
Bland Altman plots for conventional IVUS variables (Figure 3) and for individual plaque components on IVUS-VH (Figure 4) show that there were no systematic differences, for any variable, between baseline and follow-up.
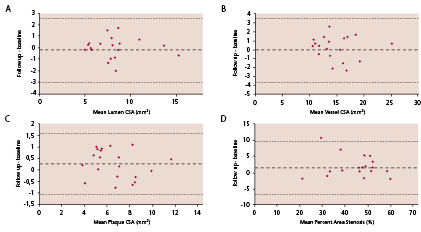
Figure 3. This Bland-Altman plot demonstrates that there is no systematic change in any conventional intravascular ultrasonography parameter
(A: lumen cross-sectional area (CSA), B: vessel CSA, C: plaque CSA and D: percent area stenosis) over the 6 month observation period.
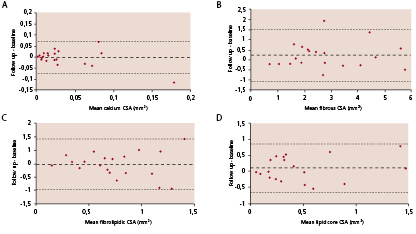
Figure 4. Panels a, b, c and d show Bland-Altman plots demonstrating that there is no systematic change in plaque composition over the 6 month observation period.
The predominant components were fibrous (68%) and fibrolipidic (20%) plaque. Typical examples of baseline and follow-up findings are presented in Figures 1 and 5.
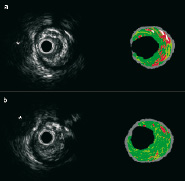
Figure 5. Matched baseline and follow-up IVUS-VH cross-sections from a patient who presented with an unheralded ST elevation myocardial infarction and had no previous medical history of note. There was a predominantly fibrous plaque (green) with islands of lipid core (red), as illustrated in a cross-sectional image (Panel A) from the baseline pullback. At six-months follow-up (Panel B), a matched cross-section (the anatomic landmark was a side-branch, indicated by the asterisk) shows a mainly fibrotic plaque with an apparent reduction in lipid core area.
Changes in IVUS-VH vs. changes in biomarkers
Temporal changes of biomarker levels are depicted in Table 4.
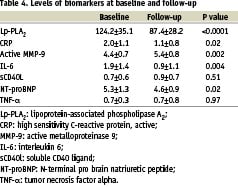
There was a significant decrease in Lp-PLA2 (p<0.0001), CRP (p=0.02), IL-6 (p=0.004) and NT-pro-BNP (p=0.02). Active MMP-9 levels increased (p=0.002). Among the seven biomarkers tested in the IBIS trial, Lp-PLA2, activity emerged repeatedly as being significantly correlated to the change in plaque composition observed by IVUS-VH analysis. As shown in Figure 6, change in lipid core area (r=0.51, p=0.024), fibrous area (r=0.49, p=0.033) and calcium area (r=0.63, p=0.004), were significantly correlated with change in Lp-PLA2.
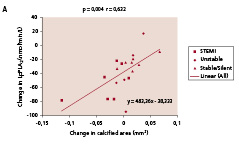
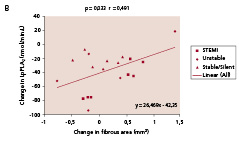
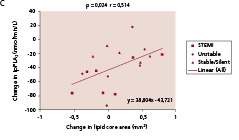
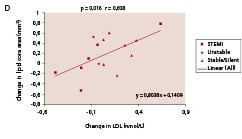
Figure 6. Significant correlations were noted between temporal changes in Lp-PLA2 activity and the changes in lipid core (panel A) fibrous component (panel B), and calcium (panel C) in mean cross sectional areas (CSA). Panel D shows the significant correlation between changes in lipid core and LDL-cholesterol. Lp-PLA2 refers to lipoprotein-associated phospholipase A2.
In parallel, the change in LDL cholesterol was also significantly correlated with the change in lipid core area (r=0.61, p=0.016, Figure 6D). In contrast, there were only non-significant trends between changes in CRP or active MMP-9 and change in lipid core area (r=0.45, p=0.061 and r=0.44, p=0.061, respectively).
Discussion
Although pathological studies have demonstrated that the potential of atherosclerotic coronary plaque to precipitate clinical events is strongly influenced by plaque composition, no imaging techniques in clinical use can reliably assess plaque composition11. In the IBIS trial, we have prospectively evaluated non-flow limiting atherosclerotic plaques with conventional and novel imaging techniques over a 6-month observational period in order to establish the background changes in the imaging parameters that reflect the variability and reproducibility of the measurements in a contemporary patient population treated with conventional medications such as statins, angiotensin-converting enzyme inhibitors and antiplatelet drugs.
The major finding of this study was that, over this relatively short observational period, there were no significant changes in the mean values of either classic IVUS parameters (vessel, lumen, or plaque area, or percent area obstruction) or of novel IVUS-VH parameters. Most IVUS studies on plaque progression have mandated follow-up at a time-point between 12 and 18 months7-10,22. The absence of significant changes on classic IVUS parameters, in the present study, is therefore not unexpected. As this is the first prospective study to assess serially plaque composition, using IVUS-VH technique, these findings must be interpreted as preliminary. The lack of significant change in plaque composition, over a short time-period suggests that the technique is reproducible in vivo. Although there were no significant changes in either mean values of classic IVUS parameters or individual plaque components on IVUS-VH, there was a trend towards an increase in plaque area, reflecting a non-significant increase in lipid core area. This observation is consistent with histopathological studies showing that the atheromatous component of plaque enlarges in a linear fashion with increasing degrees of cross-sectional narrowing and with the fact that the lipid core is the most active component of the plaque23,24. In that respect, it is remarkable to note that the majority of the relationships between the biomarkers and the compositional nature of the plaque concern the lipid core and among the biomarkers analyzed Lp-PLA2 exhibits the most active role. This enzyme plays a key role in mediating the hydrolysis of oxidatively modified phosphatidylcholines in LDL, thus generating lysophosphatidylcholine and oxidized fatty acids, molecules that are implicated in several detrimental effects in the vessel wall25-28. To what extent the observed changes in plaque composition are mediated by Lp-PLA2 or its carrier, LDL cholesterol, can be only addressed in the future larger studies with potent Lp-PLA2 inhibitors. It is noteworthy, however, that the change in levels of LDL was also positively correlated with changes in lipid core areas. These findings make the observed association between imaging-derived assessment of lipid core and both LDL (i.e., atherogenic substrate) as well as Lp-PLA2 activity (i.e, enzyme responsible for LDL-derived inflammatory mediators) more consistent. Aside from the lipid core and the calcified components, the sclerotic component of the vessel wall (fibrous tissue) accounted for almost 70% of plaque area composition. This observation is concordant with previously reported morphometric data from postmortem studies23, thus providing indirect evidence for the validity of the technique.
Plaque characterization in vivo has the potential to allow the assessment of the effects of pharmacological therapies on the coronary arteries, thereby enabling a better understanding of the disease and further development of new pharmacologic interventions. Using a slightly different approach, spectral analysis of radiofrequency data has recently shown that statins can promote detectable changes in plaque composition despite lacking a significant shift in plaque burden29. This pilot clinical study provides some indication on the boundaries of change beyond which modifications in tissue composition might be interpreted as statistically significant change in a contemporary population of patients with CAD that are treated with routine medical therapy following PCI.
Limitations
The IBIS study was an observational, non-controlled, single center study where plaque composition was re-assessed after a short time (6 months). Only a relatively short vessel segment was interrogated by IVUS (~30 mm) that may not be representative of plaque composition within the entire coronary tree. We also wish to underscore that the results of the present study are derived from a small subset of patients and will require further confirmation. Finally, the patient population included in the IBIS study was intentionally heterogeneous and most had a high level of background medication. Whether novel IVUS-based plaque imaging could refine risk stratification in patients undergoing clinically mandated cardiac catheterization will require long-term natural history studies, nonetheless, information regarding plaque composition is obtained during a standard IVUS pullback without the need for additional instrumentation of the coronary artery.
Conclusions
After a 6-month observational period, no significant changes were detected in plaque burden or composition. These results were expected, since no new therapeutic intervention was tested and all patients received routine medical care following PCI. This pilot clinical study provides some indication on the boundaries of change beyond which modifications in tissue composition might be interpreted as statistically significant. IVUS-VH may provide insights into pathophysiology in studies of the natural history of coronary plaque. Furthermore, it may provide surrogate endpoints and offers the potential to allow the assessment of emerging pharmacologic interventions with novel mechanisms of action.
Acknowledgements
This study was supported by unrestricted education grant from GlaxoSmithKline.

