Abstract
Aims: The purpose of this preclinical feasibility study was to evaluate a novel integrated platform in which magnetic navigation is used to remotely guide electromechanical mapping of the left ventricle (LV) and transendocardial cell injections. Using an integrated remote system would greatly facilitate intramyocardial delivery of stem cells for treating ischaemic heart disease.
Methods and results: We used the computer-controlled Stereotaxis magnetic navigation system to guide the NOGA electromechanical mapping system in mapping viable myocardium in the LV of seven pigs. We then tested the feasibility of this system to perform transendocardial injections in three of the pigs and to deliver mesenchymal precursor cells (MPCs) to targeted myocardial segments in four of the pigs. The success or failure of each injection was determined by myocardial contrast staining in the first group and by histopathologic analysis in the last group. The mean time (±SD) spent mapping the LV for each pig was 49.3±10.6 min. The success rate for transendocardial injections was 94.4%, as indicated by myocardial contrast staining. There was a 95.8% success rate for targeted injections of MPCs, and 4’,6-diamidino-2-phenylindole-labeled MPCs were detected in all but one segment of one pig. No epicardial haemorrhage or injury was observed, although there was some venous drainage.
Conclusions: The integrated Stereotaxis/NOGA system has excellent remote navigability inside the LV cavity while sparing the operator from radiation exposure. This system also allows transendocardial cell injections to be performed with a high success rate. Further studies are needed to define the safety profile of this system for clinical use.
Introduction
Recent studies have suggested that the heart has an intrinsic ability to regenerate after injury1-3. This new finding has stimulated research on stem cell therapy for ischaemic heart disease4-7. Preliminary preclinical data have shown that intramyocardial delivery of stem cells results in better cell retention than do intracoronary or retrograde injections through the coronary venous sinus8.
Electro mechanically guided injection is the currently preferred mode of delivery in patients with chronic myocardial ischaemia. Using the NOGA electromechanical mapping (EMM) system to deliver transendocardial injections has been shown in clinical studies to be feasible and safe4,5,9,10. The capability to perform online assessment of myocardial ischaemia and viability using unipolar voltage mapping has also been previously described11-13. Segments of myocardium with unipolar voltage less than 6.9 mV are associated with transmural scarring on MRI, with a sensitivity of 93% and specificity of 88%12. The ability of the NOGA system to obtain a detailed map of the LV cavity with 1-mm precision, combined with its ability to differentiate viable from non-viable myocardium, permits the accurate targeting of cell delivery to ischaemic or viable myocardium.
The Stereotaxis magnetic navigation system (MNS) comprises two magnets that can be oriented to generate a 0.08-T directional magnetic field. This new system has had good preliminary safety results in patients with complex anatomy undergoing percutaneous coronary interventions14. The safety and feasibility of this system for guiding electrophysiology catheters for diagnostic and interventional procedures has also been shown15,16.
Integrating the NOGA EMM system with the Stereotaxis MNS would allow remote navigation inside the left ventricular (LV) cavity. In theory, the electromagnetic force exerted at the catheter tip would preclude the need for support from its shaft, thus allowing for the use of a softer catheter that is less likely to penetrate the myocardium and cause perforation. The new catheter also allows for transendocardial injections in remote areas of the LV not easily reached with the current manually directed catheters. This remote navigation capability of this system would also avoid excessive mapping times and undue exposure of the operator to radiation.
The purpose of this preclinical feasibility study was to evaluate a novel integrated Stereotaxis MNS-guided NOGA EMM system for remote mapping and stem cell injections. We used a porcine model to test the ability of this system to construct a LV electromechanical map and to deliver transendocardial injections to various myocardial segments.
Methods
Stereotaxis MNS-guided NOGA EMM system
The MNS (Niobe®, Stereotaxis, St. Louis, MO, USA) consists of two computer-controlled permanent magnets, located in a fixed housing apparatus, that can be manoeuvred relative to each other to create a directional magnetic field16. The apparatus is located on either side of the fluoroscopy table (AXIOM Artis, Siemens, Malvern, PA, USA) and can be adjusted to the patient’s body position. While in the “navigate” position, the magnets create a relatively uniform magnetic field (0.08 T) of approximately 15 cm in diameter inside the patient’s chest.
The Stereotaxis-compatible NogaStar® mapping and MyoStar® injection catheters (Biologics Delivery Systems, Cordis Corporation, Diamond Bar, CA, USA) are equipped with a small permanent magnet positioned at the tip that aligns itself with the direction of the externally controlled magnetic field, thereby enabling the catheter to be steered effectively. Changing the orientation of the outer magnets relative to each other changes the orientation of the magnetic field, leading to deflection of the catheter17 (Figure 1).
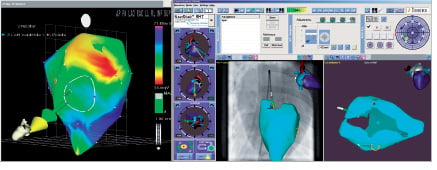
Figure 1. Mapping the LV cavity with the NogaStar® catheter. Left: unipolar voltage map of the LV cavity, showing the Stereotaxis-compatible NogaStar® catheter, the vector of the catheter position (green), and the directional vector (yellow) created by the magnetic field. Right: Navigant II screen showing integration of the fluoroscopy image and the endocardial map.
All magnetic field vectors can be stored and, if necessary, reapplied while the magnetic catheter is navigated automatically. In addition, a computer controlled catheter advancer system (Cardiodrive unit, Stereotaxis, St. Louis, MO) is used to allow truly remote catheter navigation without the need for manual manipulation after the catheter has been inserted into the ascending aorta. The video workstation (Navigant II, Stereotaxis, St. Louis, MO, USA), in conjunction with the Cardiodrive unit, allows precise orientation of the catheter by 1° increments and by 1-mm steps in advancement and retraction. The system is controlled by joystick or mouse and allows remote control of the injection catheter from inside the control room, away from radiation sources.
The NOGA system enables users to identify viable myocardium by assessing unipolar voltage12. Thus, integrating the NOGA XP (Microsoft Windows platform) with the Stereotaxis MNS would allow for remote LV navigation and targeted stem cell delivery into viable ischaemic myocardium. The integration of the two systems at the level of the user interface is achieved by simply placing a three dimensional vector into the NOGA map that can be moved by clicking and dragging a computer mouse. Catheter movement tracks the orientation of the vector. No catheter manipulation is required.
Animals
This study was reviewed and approved by the Institutional Animal Care and Use Committee of Washington University (St. Louis, MO, USA) and met the criteria of the National Institutes of Health and American Heart Association for animal research. Seven healthy adult domestic pigs of either sex, weighing between 30 and 55 kg, were subjected to cutdown exposure of the femoral artery for catheter insertion, performance of the EMM procedure, and transendocardial injections.
Remote electromechanical mapping procedure
In brief, general anaesthesia was induced with intravenous pentothal (17 mg/kg) and maintained with isoflurane (1.5% to 2.25%) in each pig. The pig’s right femoral artery was then exposed, an 8F introducer was positioned, and the NogaStar® catheter was manually advanced into the ascending aorta. The Cardiodrive unit was used to advance the catheter though the aortic valve into the LV cavity while orienting the magnetic field vector posterosuperiorly to bend the catheter tip against the valve leaflets. After the catheter entered the LV cavity, the vector was redirected to the apex, and the catheter was advanced until it contacted the endocardial surface. Electromechanical mapping was performed by orienting the vector and advancing or retracting the catheter with the Cardiodrive unit until a complete map of the LV cavity was acquired.
Transendocardial injections
The prototype magnetic injection catheter (Stereotaxis-compatible MyoStar®) was evaluated for its navigational characteristics, magnetic deflection capabilities, and the amount of push force exerted by the catheter tip. In a previous study, a prototype injection catheter showed good success in navigating a Stereotaxis small heart phantom; the catheter was able to reach all targets of interest in the LV via the retrograde approach. The injection catheter prototype catheter tip could also be deflected by 150° when the unsupported (or freely deflectable) length was 5 cm. This is comparable to the tip deflection of 132° obtained with the MyoStar® catheter with a similar free length. The push force exerted by the injection prototype catheter just before the catheter tip buckles, unaided by any magnetic field, was 70 g when the unsupported length was 4.5 cm. Under similar conditions, the tip force exerted by the MyoStar® catheter overloaded the test gauge (> 100 g) without any buckling of the catheter tip. The maximum tip force exerted by the prototype catheter decreases progressively as the tip is bent in response to the applied magnetic field. This will likely contribute to added safety during transendocardial injections in regard to avoiding myocardial perforation (Figure 2).
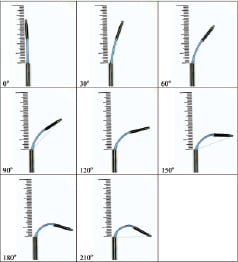
Figure 2. Magnetic deflection of the prototype catheter. Images show magnetic deflection of a prototype catheter in various directions in a 0.08-T field when the catheter is extended 4.5 cm from rigid support.
Before performing the transendocardial injections, we manually divided the EMM into a polar map with 13 segments, excluding the most basal segment of the heart (Figure 3).
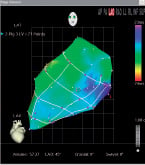
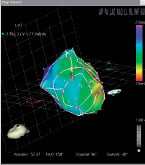
Figure 3. Manually reconstructed polar map from one representative animal of phase I of the study. The basal segments were excluded.
The NogaStar® catheter was replaced with the MyoStar® catheter, and the needle length was set at 5 mm.
Phase I of the study tested the feasibility of using the integrated Stereotaxis/NOGA system to perform transendocardial injections. In three pigs, each of the 13 predefined segments was targeted and injected with 0.2 ml of isosmolar contrast medium (iodixanol [Visipaque], Amersham Health, Princeton, NJ, USA). Each segment received three injections of contrast medium. In the second animal the apical segments received additional nine injections. The presence of contrast stained myocardium by fluoroscopy confirmed the success or failure of each transendocardial injection. For each injection, the catheter angle relative to the endocardial surface was recorded as well as the force exerted by the catheter tip against the endocardium (measured as quartiles from 0 [minimum force] to 4 [maximum force]), and the presence of premature ventricular contraction (PVC) upon needle exposure.
Phase II of the study tested the ability of the integrated Stereotaxis/ NOGA system to deliver bone marrow derived allogeneic mesenchymal precursor cells (MPCs; Angioblast Systems) to targeted segments. The first stem cell-injected animal received unlabelled MPCs and the three animals that followed were injected with 150 million 4’,6-diamidino-2-phenylindole (DAPI)-labeled MPCs diluted in 4.8 cc of phosphate buffered saline. In all four animals the injections were targeted to the mid-ventricular segments (anterior, anteroseptal, inferoseptal, inferior, inferolateral, and anterolateral). Four stem cell injections of 0.2 cc were delivered to each predefined segment. As in phase I, we recorded for each injection the catheter angle relative to the endocardial surface, the force exerted against the endocardium (measured as quartiles from 0 to 4), and the presence of PVC upon needle exposure. The pigs were euthanised after the procedure, and their hearts were sent for histopathologic analysis.
Histopathologic analysis
After each animal was euthanised, the aortas and hearts were rapidly excised. Shortly after excision, the pericardial sac of each phase I and II study animal was examined for signs of effusion, and each heart was evaluated for epicardial haemorrhage or injury. Additionally, in the phase II animals, each heart was also evaluated for the presence of DAPI-labelled cells as follows. The heart was perfusion-fixed and then sliced into 1-cm-thick sections (bread-loaf technique). Each slice was photographed on both sides. A portion of the slices was then placed in 10% buffered formalin for immediate histological evaluation, and the other portion was frozen in Tissue Tek optimal cutting temperature compound (Sakura Finetek USA, Torrance, CA, USA) and stored at – 80°C for subsequent histologic analysis. The histopathology slides were correlated with the correspondent injected segment to detect DAPI-labeled MPCs by fluorescence microscopy. Needle tracks were identified, and the depth of each injection (vertical distance from the endocardial surface) was calculated.
Statistical analysis
Descriptive statistics were performed using SPSS software (standard version 11.0, SPSS). Numerical variables are reported as mean±SD. Categorical data are reported as proportions.
Results
The integrated Stereotaxis/NOGA system was used to perform remote EMM and transendocardial injections in seven pigs. The weight of the animals, the number of map points per animal, and the time spent for the mapping and injection procedures are summarised in Table 1.
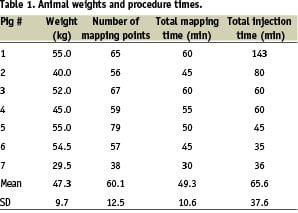
The mean mapping time (±SD) was 49.3±10.6 min per animal, or 49.4±6.3 s per mapping point.
Phase I: injection feasibility
A total of 126 0.2-ml injections of iodinated contrast dye were performed. One hundred and nineteen injections (94.4%) were successful; that is, they resulted in myocardial contrast staining. The presence of PVC upon needle exposure predicted successful transendocardial injection with a sensitivity of 98% and a specificity of 71%. The catheter angle relative to the endocardial surface varied from < 30° to 90°, and the force exerted against the endocardium varied on a scale of 0 to 4. Transendocardial injections had a high rate of success regardless of the catheter angle and force (Table 2).
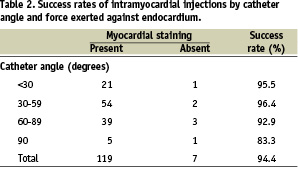
Venous drainage, defined as contrast staining of the vein, was observed in 50 (39.7%) of the 126 injections. Histopathologic analysis revealed no evidence of pericardial effusion, epicardial haemorrhage, or injury.
Phase II: Stem cell injection feasibility
A total of 96 injections of MPCs were performed. In the first animal injected with unlabelled MPCs, the presence of MPC clusters and corresponding needle tracks in the myocardium was confirmed by gross microscopy (Figure 4).

Figure 4. Transendocardial injections of unlabelled MPCs. (A) Several needle tracks (arrows) and an area of micro-haemorrhage (box) in the subepicardial space. (B) Distribution of MPCs along a needle track, with concomitant haemorrhage (red areas). (C) Cluster of MPCs at higher magnification.
In the remaining three animals that received transendocardial injections of DAPI-labelled MPCs, the presence of MPCs was assessed under fluorescence microscopy. The depth of the injections was also assessed at higher magnification.
MPCs were detected in 23 of the 24 injected segments for each animal, comprising a success rate of 95.8% (Figure 5).
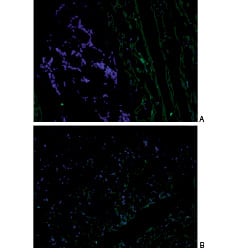
Figure 5. Frozen sections of an injected myocardial segment imaged under fluorescence microscopy (blue, DAPI-labelled nuclei; green, α-sarcomeric actinin). A. Small cell cluster corresponding to an injection site. B. DAPI-labelled MSCs dispersed throughout the apex. Magnification: x20.
Histo-pathologic analysis revealed no evidence of pericardial effusion. In each animal, needle tracks could be identified in 18 of the 24 injected segments. In 16.7% of injected segments, the needle track length reached the entire thickness of the correspondent myocardial segment. The remaining needle tracks identified injections with a mean depth of 53.5±17.3% of the LV wall thickness.
The only injected segment (out of 24 injected segments) that did not contain injected cells was the mid-inferior segment of pig #7. In this pig, DAPI-labelled cells were found at the epicardial surface, and small petechiae were found in the mid-anterior segment (Figure 6).
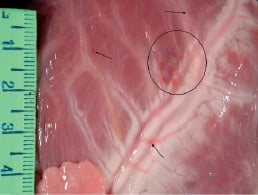
Figure 6. Small petechiae (arrow and circled area) in the anterior wall of pig #7.This pig had a substantially lower body weight, a lower heart weight (235 g), and thinner LV walls than did the other animals.
Discussion
This initial preclinical experience provides preliminary evidence of the feasibility of remote EMM, transendocardial injections, and LV cavity navigation with the integrated Stereotaxis MNS-guided NOGA system. The new Stereotaxis-compatible mapping (NogaStar®) and injection (MyoStar®) catheters have demonstrated excellent capacity for intraventricular navigation, being able to reach every endocardial segment needed to construct an electromechanical map and successfully perform endocardial injections. Furthermore, this system preserved navigation precision and map quality while sparing the operator from exposure to radiation during the procedure.
Magnetically driven navigation has important advantages over conventional catheter manipulation inside the LV. Because the force driving the catheter and holding it stable against the endocardium is exerted on the tip of the magnetically guided catheter, its shaft does not need to provide backup support and is flexible enough to allow the movement guided by the magnetic field. This feature permits the catheter to bend at acute angles to reach remote segments while keeping a stable position after needle exposure. This allows for easy mapping and injection of LV segments, such as the high anterior wall, that are extremely difficult to reach with conventional catheter techniques. The excellent navigability of the catheter and the magnetic force holding it against the myocardium could explain the high success rate of our transendocardial injections (Figure 7) regardless of the catheter angle or its force against the endocardium.
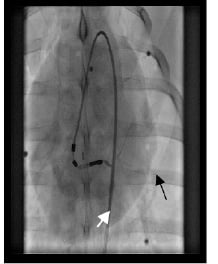
Figure 7. Image from fluoroscopy of a transendocardial injection, showing the acute angle made by the catheter. Myocardial staining confirms the intramyocardial position of the needle. Vein drainage is evident (arrow).
In addition, the softer shaft of the magnetically guided catheter may help prevent cardiac perforation.
The present study offers new insights into transendocardial injections. First, we found that the presence of a PVC upon needle extension can be used to guide transendocardial injections. In the first set of transendocardial injections, the presence of PVC predicted the success of the injections with high sensitivity. Second, a substantial number of contrast injections (nearly 40%) resulted in some degree of drainage by the coronary venous system. This phenomenon could influence retention rates after delivery of cells though this route, so transendocardial delivery strategies may need to be optimised to limit venous delivery of cells.
Study limitations
Because of the small number of animals and the constant needle length used in this study, the full safety profile of this new catheter navigation system could not be established. Although there was no histopathologic evidence of pericardial effusion, injected cells were found in the epicardium of one pig, which also had petechiae in the epicardial surface. Further preclinical studies specifically designed to assess safety should be performed before this system is used for transendocardial injections in patients.
The success rates with this new catheter may have been overestimated because of the small number of animals used, although we did perform a large number of injections that were well distributed throughout the LV. In addition, the success rates achieved with this new catheter have not been compared head-to-head with those achieved with conventional manual navigation.
Conclusions
The integrated Stereotaxis MNS-guided NOGA EMM system can be used for remote navigation inside the LV cavity and allows the performance of transendocardial injections with a high success rate. The feasibility of this system for transendocardial cell delivery was confirmed by histopathologic analysis. More studies are needed to define the safety profile of this system.
References

