Abstract
Aims: Although safety and feasibility studies have been published, there are few reports dedicated to the echocardiographic evaluation of patients following percutaneous aortic valve replacement (PAVR). This report describes the early echocardiographic evaluation of patients undergoing PAVR with the CoreValve Revalving System.
Methods and results: The population consisted of 33 consecutive patients with aortic stenosis who underwent successful PAVR. Echocardiograms were performed pre-treatment (123±110 days prior), post-treatment (6±2 days) and post-discharge (80±64 days). Aortic valve function and left ventricular dimensions, systolic and diastolic function were assessed pre- and post-implantation. The mean age was 81±7 years and the mean Logistic Euroscore was 20±12. Following PAVR, the mean transaortic valve gradient decreased (46±16 mmHg pre-treatment vs. 12±7 mmHg post-treatment vs. 9±5 mmHg post-discharge, p<0.001) and the mean effective orifice area increased (0.75±0.23 cm2 pre-treatment vs. 1.97±0.85 cm2 post-treatment vs. 1.72±0.45 cm2 post-discharge, p<0.001). There was no significant change in mean ejection fraction (41±12% pre-treatment vs. 46±15% post-treatment vs. 44±13% post-discharge, p=0.44). Approximately two-thirds of patients had no change in diastolic function at follow-up.
Conclusion: Following implantation, there was a sustained decrease in aortic valve gradient and increase in aortic valve area. In addition, the mean ejection fraction did not change significantly and in the majority of patients, diastolic function was unchanged.
Introduction
Transcatheter aortic valve replacement has become regarded as a viable alternative to surgical aortic valve replacement in elderly patients with severe aortic stenosis who are considered too high a risk or denied surgery. Up until now, reports on transfemoral percutaneous aortic valve replacement have consisted mainly of safety and feasibility studies demonstrating a significant improvement in haemodynamic and clinical status1-8. Procedural-related and 30-day mortality rates after PAVR is reported to be 6% and 12%, respectively, while freedom from death, myocardial infarction or stroke at 30 days ranges from 74 - 86%3,6,7.
Echocardiography is the gold standard for the evaluation of pre- and post-surgical aortic valve replacement9. Following surgical aortic valve replacement, acute and long-term improvements in aortic valve gradients, regression in left ventricular mass and improvement in left ventricular diastolic function is reported11-21. There are, however, few reports dedicated on the echocardiographic evaluation post PAVR22.
In this study, we report on the early echocardiographic evaluation of patients undergoing PAVR with the CoreValve Revalving System (CRS) derived from an independent core laboratory (Cardialysis, Rotterdam, The Netherlands).
Methods
Patients
Thirty-three consecutive patients undergoing implantation with the CRS between November 2005 and December 2007 at the Thoraxcenter, Rotterdam were included in the analysis. Patients referred for the procedure were deemed either too high or prohibitive risk for surgical aortic valve replacement. Patients were treated in the framework of the CoreValve safety and feasibility protocols (COR 2005, COR 2006-02) and the CE post marketing surveillance registry.
The inclusion and exclusion criteria have been described elsewhere7. Briefly, patients were included if they had (1) severe native aortic valve stenosis with an area <1 cm2 or <0.6 cm2/m2 with or without aortic regurgitation; (2) aortic valve annulus diameter > 20 mm and < 27 mm and (3) sinotubular junction < 43 mm measured by echocardiography.
Patients provided written consent after a consensus was achieved between a cardiologist and a cardiac surgeon that surgical aortic valve replacement was associated with either too high or prohibitive risk.
Device description and procedure
Descriptions of the device and technical aspects of the procedure have been previously published23. The CoreValve aortic valve prosthesis consists of a self-expanding nitinol tri-level frame to which a trileaflet bioprosthetic porcine pericardial tissue valve is mounted and sutured.
The initial two procedures were performed under femoral-femoral circulatory support and a total of nine procedures were performed using the Tandem Heart (left atrial-to-femoral artery bypass system). The remaining 22 patients underwent a completely percutaneous procedure with echo-assisted vascular access and percutaneous closure with a 10 Fr Prostar XL device23. Prior to the implantation of the prosthesis, percutaneous aortic balloon valvuloplasty was performed using rapid ventricular pacing. Device positioning and deployment was performed under fluoroscopic guidance only. Five patients (15%) were implanted with the 2nd generation 21 Fr catheter system and 28 patients (85%) were implanted with the 3rd generation 18 Fr catheter system. Twenty-six patients (79%) were implanted with the 26-mm inflow prosthesis and when it became later available, seven patients (21%) were implanted with the 29-mm inflow prosthesis. A total of nine patients had moderate-severe aortic regurgitation immediately after implantation of the prosthesis. As a result, five of these patients underwent re-dilatation with balloon valvuloplasty and the remaining four patients received a 2nd CRS during the index procedure.
Echocardiographic assessment
A standard 2-D transthoracic echocardiogram (Philips Ie33 or Sonos 7500, Philips, Best, The Netherlands) was analysed pretreatment, post-treatment and early post-discharge. Two independent cardiologists reviewed the echocardiograms.
Echocardiographic studies were performed in a standard fashion. Quantification of left ventricular end-systolic and end-diastolic volumes and ejection fraction was performed using the biplane Simpson’s method. Left ventricular end-systolic and end-diastolic dimensions (mm), interventricular septal and left posterior wall thickness (mm) and left atrial size (mm) measurements were obtained using the parasternal long axis view by either M-mode or 2-D echocardiography.
Diastolic function was assessed by pulsed-wave mitral inflow patterns and tissue Doppler imaging of the mitral annulus according to standard recommendations. Diastolic function was classified as normal, mild diastolic dysfunction (impaired relaxation), moderate diastolic dysfunction (pseudo-normal) and severe diastolic dysfunction24.
The diameters of the left ventricular outflow tract and aortic annulus were obtained by standard 2-D echocardiographic calliper measurement from the parasternal long axis view. Velocity-time integral (cm), peak aortic velocity (cm/sec), peak instantaneous gradient (mmHg) and mean transaortic gradient (mmHg) were measured. By using the modified Bernoulli equation (dP=4v2), a peak instantaneous valve gradient (mmHg) was derived from the continuous-wave Doppler velocity across the aortic valve. The effective orifice aortic valve area (cm2) was estimated by the continuity equation.
Colour-flow Doppler and continuous wave Doppler signal was used to quantitate aortic regurgitation9,10. Post-procedural aortic regurgitation was further classified as either central or paravalvular in origin. Mitral regurgitation was quantified using colour and continuous wave Doppler flow. Central valvular insufficiency was graded as none, mild, moderate or severe9,10.
Left ventricular mass index (g/m2) was estimated using the formula proposed by Devereux and colleagues: 0.8 (1.04 ([LVIDD + PWTD + IVSTD]3 – [LVIDD]3)) + 0,6 g (where, LVIDD=left ventricular internal diameter, PWTD=posterior wall thickness at end-diastole, IVSTD=interventricular septal thickness at end-diastole)25.
Statistical analysis
Categorical variables are presented as frequencies and continuous variables are presented as mean±standard deviation. A one-way ANOVA test was used for comparison between groups. A p value of < 0.05 was considered statistically significant.
Results
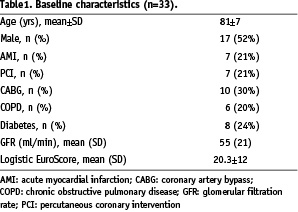
The baseline patient characteristics are summarised in Table 1.
Successful valve implantation was achieved in all patients. One patient died six days after PAVR as a consequence of procedural-related cardiac tamponade. Another patient died at 51 days related to sepsis. The completeness of the echocardiographic follow-up is shown in Figure 1.
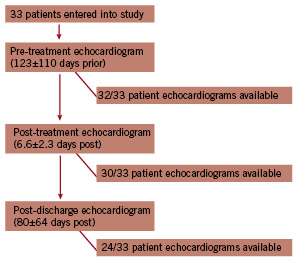
Figure 1. Flow diagram depicting time of follow-up and number of available echocardiograms at each time period.
Patients with missing echocardiograms were all alive. The reason for missing echocardiograms included one of the following: (1) poor acoustic windows rendering the echocardiogram not suitable for interpretation; (2) follow-up care was reassigned to the referring hospital; or (3) the patient had not reached the follow-up time point.
Pre-treatment echocardiographic evaluation
Pretreatment echocardiographic analysis was available in 32 out of the 33 patients (Figure 1). The echo study was performed at a mean of 123±110 days prior to PAVR. The findings are summarised in Table 2.
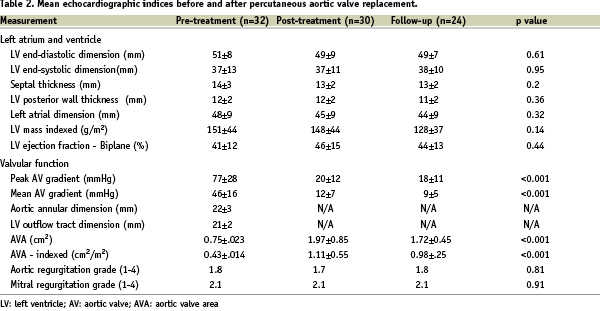
The left ventricular end-diastolic and end-systolic dimensions were above the normal range (> 56 mm and > 40 mm, respectively) in 34% and 39% of patients, respectively. The septal thickness and left ventricular posterior wall thickness was above the normal range (> 12 mm) in 68% and 65% of patients, respectively. No patient had an interventricular septum-to-posterior wall thickness ratio greater than 1.3, therefore excluding asymmetric hypertrophic cardiomyopathy.
All patients had a pre-treatment aortic valve area index < 0.6 cm2/m2 (mean±SD, 0.43±0.14 cm2/m2). Five patients had an ejection fraction < 30%.
The frequencies of aortic and mitral regurgitation are depicted in figure 2 and 3, respectively.
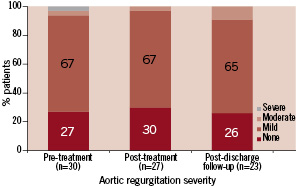
Figure 2. Severity of aortic regurgitation during serial echocardiographic evaluation (n=number of patient echocardiograms with sufficient information for assessment of aortic regurgitation).
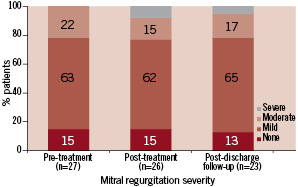
Figure 3. Severity of mitral regurgitation during serial echocardiographic evaluation (n=number of patient echocardiograms with sufficient information for assessment of mitral regurgitation).
Classification of diastolic function was feasible in 27/32 patients and is summarised in Figure 4.
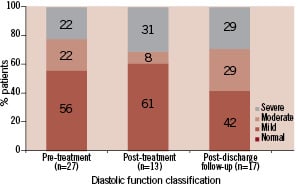
Figure 4. Classification of diastolic function during serial echocardiographic evaluation (n=number of patient echocardiograms with sufficient information for assessment of diastolic function).
The mean left ventricular mass index was increased in 84% of patients (normal range, male 49-115 g/m2 and female 43-95 g/m2).
Post-treatment echocardiographic evaluation
Echocardiographic evaluation was performed at a mean of 6±2 days following PAVR and was available in 30 out of the 33 patients (Table 2).
There was an immediate reduction in mean transaortic valve gradient from 46±16 to 12±7 mmHg and an increase in the estimated mean effective aortic orifice area from 0.75±0.23 to 1.97±0.85 cm2 (Figure 6 and 7).
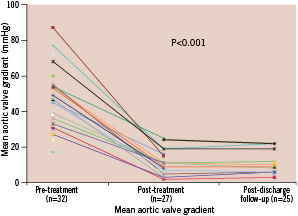
Figure 6. Mean aortic valve gradient (mmHg) during serial echocardiographic evaluation (n=number of patient echocardiograms with sufficient information for assessment of mean aortic valve gradient).
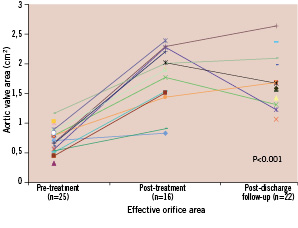
Figure 7. Effective orifice valve area (cm2) during serial echocardiographic evaluation (n=number of patient echocardiograms with sufficient information for assessment of effective orifice valve area).
The severity of aortic regurgitation post-treatment is shown in Table 2 and Figure 2. Figure 5 provides individual patient information regarding the change in aortic regurgitation.
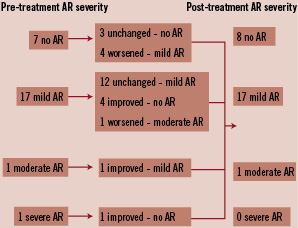
Figure 5. Descriptive change in aortic regurgitation grade from baseline to discharge (n=26 patients with both pre-treatment and post-treatment aortic regurgitation severity available for comparison).
After PAVR, 23% of the patients had an improvement in the degree of aortic regurgitation 19% had a worsening. Aortic regurgitation was paravalvular in all patients except two in whom there was concomitant central aortic regurgitation. All patients who required either a re-dilatation with balloon valvuloplasty (n=5) or a valve-in-valve implantation (n=4) had mild paravalvular aortic regurgitation post-treatment.
The severity of mitral regurgitation post-treatment is shown in Table 2. It remained unchanged in 65%, improved in 12%, and worsened in 23% of the patients (Figure 3). In those patients who worsened, two had mild, two had moderate and one had severe mitral regurgitation.
After implantation of the prosthesis, the mean ejection fraction remained unchanged (Table 2).
Classification of diastolic function was possible in 13 patients (Figure 4). Of the 12 patients with pre- and post-treatment evaluation of diastolic function, there was no change in diastolic function in eight patients (66%) (Figure 8).
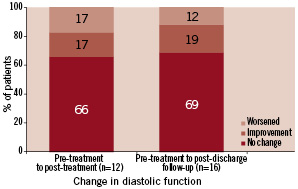
Figure 8. Intra-individual change in diastolic function during serial echocardiographic evaluation from pre-treatment to post-treatment and from pre-treatment to post-discharge follow-up (n=number of patient echocardiograms with sufficient information for assessment and comparison of diastolic function).
Post-discharge echocardiographic evaluation
A post-discharge echocardiogram was available in 24/33 patients (73%) and was performed at a mean of 80±64 days post PAVR (Table 2).
The reduction in the mean aortic valve gradient was maintained at follow-up (9±5 mmHg) (Figure 6) as well as the increase in the mean effective aortic orifice area (1.72±0.45 cm2) (Figure 7).
The mean aortic regurgitation grade did not change at post-discharge follow-up (Table 2). On an individual basis, aortic regurgitation did not change in 37% of the patients, improved by 1 grade in 37% and worsened by 1 grade in another 36%. No patient had severe aortic regurgitation (Figure 2). In all patients, aortic regurgitation was paravalvular.
The degree of mitral regurgitation during follow-up did not change in 44% of the patients, it improved in 33% improved and worsened in 23%.
There was no significant change in ejection fraction (Table 2).
Classification of diastolic function at follow-up is shown in Figure 4. In the majority of patients (69%), diastolic function did not change compared to baseline classification (Figure 8).
There was no statistically significant change in left ventricular mass index at a mean of 80±64 days following PAVR (151±44 g/m2 pre-treatment vs. 128±37 g/m2 post-discharge, p=0.14).
Discussion
The results of this study demonstrate a significant decrease in aortic valve gradient and increase in valve area post PAVR. Yet, mild paravalvular aortic regurgitation after PAVR was present in the majority of patients (65%). There was no significant change in mean left ventricular ejection fraction. In addition, we found no significant change in diastolic function early after PAVR.
Transaortic valve gradient and effective orifice aortic valve area
The changes in gradient and valve area observed in this study are consistent with the findings of others. Grube et al reported a reduction in the mean aortic gradient from 43.7 mmHg to 9.0 mmHg (p<0.001) following the implantation of the CRS in 76 patients7. Webb et al found a reduction in the mean aortic gradient from 46±17 mmHg to 11±5 mmHg following the implantation of a balloon-expandable stent valve and an increase in AVA from 0.6±0.2 cm2 to 1.7±0.4 cm2.6 Cribier et al demonstrated a reduction in the mean aortic gradient from 37±13 mmHg to 9±2 mmHg and an increase in the mean AVA from 0.60±0.09 cm2 to 1.7±0.11 cm2.5 These results compare favourably to surgical aortic valve replacement whereby, following replacement with a bioprosthetic Carpentier-Edwards valve, the echocardiography-derived mean gradient is reported to be 14±6 mmHg and AVA 1.8 cm2.26
Paravalvular aortic regurgitation
In this study, mild paravalvular aortic regurgitation after PAVR was found in 67% of the patients. Although some had moderate to severe regurgitation immediately following PAVR, this was corrected by re-dilatation or the implantation of a second valve. These patients for whom further re-intervention was required had mild paravalvular aortic regurgitation on post-treatment follow-up. Importantly, no patient had severe regurgitation. In the study by Grube et al, re-dilatation was performed in 21 out of the 86 patients and a second valve was implanted in two patients8. Aortic regurgitation after PAVR is nearly always paravalvular and may be explained by inadequate sizing of the valve due to inadequate annulus size measurement before PAVR or the lack of sufficient ranges of frame sizes, insufficient expansion of the frame due to the presence of calcium in the aortic root and leaflets, malpositioning of the valve (in most cases, too low), and leaflet malcoaptation which leads to central regurgitation. It must be recognised that there is currently no systematic or recommended method to adequately grade or characterise paravalvular aortic regurgitation. In addition, the results could have been different were transesophageal echocardiography had been used.
In 20-30% of patients, paravalvular aortic regurgitation improved or worsened during the short period of follow-up. The changes, however, were minimal. These observations are in parallel with those of others2-4. The changes in regurgitation may be related to the variability in repeat echo-Doppler studies and analyses, variable changes in the left ventricular outflow tract geometry after PAVR, recoil and/or further expansion of the nitinol frame. Aortic regurgitation may also be caused by dysfunction of the frame due to corrosion of the nitinol, leading to strut fracture27. This is unlikely in the present series due to the short follow-up.
Left ventricular ejection fraction
We found no change in the left ventricular ejection fraction. These observations, in addition to being limited by the small sample size, may also be due to concomitant coronary artery disease and scar formation from old myocardial infarctions. In addition, no patient had worsening of ejection fraction of clinical relevance. Webb et al and Cribier et al found a significant increase in ejection fraction post-treatment from 53±15% to 57±13% and from 45±18% to 53±14%, respectively4,6. Not surprisingly, the improvement in left ventricular function in these studies was predominantly seen in patients who had moderate to severe left ventricular dysfunction at baseline. Similar observations have been observed after surgical aortic valve replacement10.
Diastolic function
The majority of patients (69%) had no change in diastolic function and no patient had normalisation of diastolic function. The small sample size precludes firm conclusions. Diastolic dysfunction, however, is a determinant of poor cardiovascular outcome28. In surgical series, changes in diastolic function have been documented within two weeks following surgical aortic valve replacement29. These early changes have been attributed to a reduction in left atrial pressure but other factors, such a medication, volume status and loading conditions may also play a role. The longer-term improvements in diastolic function are likely an expression of the regression in left ventricular hypertrophy29.
Limitations and conclusion
The present study is limited by the small sample size and short duration of follow-up. In addition, echocardiographic follow-up was not complete and the quality of certain frames precluded analysis of certain variables. Furthermore, potential differences in systolic or diastolic function could possibly exist between the patient cohorts treated with or without circulatory support, but the small sample size would prohibit any reasonable conclusions. Short and long-term prospective echocardiographic studies are required.
These studies may help to determine predictors of procedural and clinical outcomes. They may also evoke recommendations for improvements in the technology itself.

