Abstract
Aims: REvascularization in Ischaemic HEart Failure Trial (REHEAT) is a registry prospectively evaluating the outcomes of percutaneous myocardial revascularization in postinfarction patients with ischemic cardiomyopathy and various categories of surgical risk.
Methods and results: One hundred seventy consecutive postinfarction patients with LVEF <40% and angiographically documented coronary stenoses eligible for PCI were enrolled to the study. The study end-points included: angiographic success of PCI, major adverse events at 30 days and 1 year after procedure, long-term survival, functional status (CCS and NYHA class) and LVEF 12 months after the intervention.
Angiographic success rate was 98,8% and complete revascularization was achieved in 38.8% cases. No periprocedural deaths were registered. Thirtieth-days survival was 97% and was better in comparison to calculated survival for CABG patients; 1-year survival was 94.4% and was not inferior to predicted survival after CABG.
In the general study population a significant improvement of LVEF (27,8±7,0 to 35,9±9,4%) was shown (absolute change mean 6.45±10%). In low/intermediate risk group the LVEF increase was lower (6.5±10,9) in comparison to high risk group (10,3±9,6%)(p=0,042). In both groups a significant and comparable reduction of angina and heart failure severity was shown in 1-year follow-up.
Conclusion: PCI in postinfarction patients with markedly reduced LVEF is associated with a significant increase of LVEF and favorable clinical outcome (CCS and NYHA class). PCI is safe, feasible and can be an alternative approach to CABG both in low/intermediate and high surgical risk patients.
Abbreviations
PCI = percutaneous coronary intervention
LVEF = left ventricular ejection fraction
CABG = coronary artery bypass grafting
Introduction
Myocardial revascularization using CABG improves survival in patients with ischemic heart disease in comparison to medical therapy as shown in controlled randomized clinical trials (CASS, VCS, ECSS)1-3. Surgical revascularization is feasible and effective in patients with ischemic cardiomyopathy and low left ventricular ejection fraction (LVEF), however it is associated with a substantial risk of periprocedural complications4. Percutaneous coronary intervention (PCI) with stent implantation is presently widely used. It is less invasive than surgical treatment and offers predictable angiographic outcomes, high success rate and low complications rate. Moreover, the use of modern antiplatelet regimens substantially reduces the risk of postprocedural myocardial infarction as well as acute and subacute in-stent thrombosis5. So far none of the randomized clinical trials comparing the outcomes after CABG and PCI aimed specifically to assess the relative efficacy of these two approaches in patients with ischaemic heart disease and low left ventricular ejection fraction. Moreover, there are limited data on late outcome of PCI in this group of patients.
Aim
The aim of this study was to prospectively assess the outcomes of percutaneous myocardial revascularization in postinfarction patients with ischemic cardiomyopathy, low left ventricular ejection fraction and various categories of surgical risk.
Patients and methods
Study population and subgroups
The study population consisted of 170 patients with postinfarction ischaemic cardiomyopathy, moderate or severe angina (CCS class II,III or IV), LVEF <40% and angiographically documented coronary stenoses eligible for percutaneous revascularization. Additional inclusion criteria were: myocardial ischemia documented on rest ECG or treadmill stress-test and/or documented viable myocardium in the area supplied by one of the target vessels with dobutamine stress echo or thallium scintigraphy.
Following exclusion criteria were used:
– known contraindications for antiplatelet therapy due to aspirin or thienopiridine allergy, bleeding diathesis, active gastrointestinal bleeding, hemorrhagic stroke,
– any stroke within 30 days,
– myocardial infarction within 14 days,
– valvular heart disease requiring surgical correction,
– predicted more complete revascularization with CABG at similar risk
– coexisting diseases associated with expected survival shorter than 12 months,
– denial of informed consent for percutaneous procedure and/or study involvement.
The patients were enrolled between January 1997 and October 2003 in Upper Silesian Cardiology Center in Katowice. Clinical and demographical characteristics of the study population is shown in Table 1.
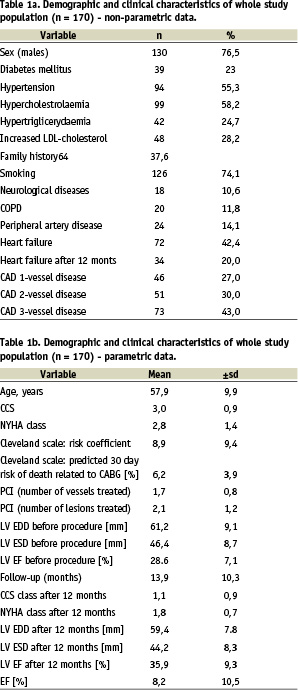
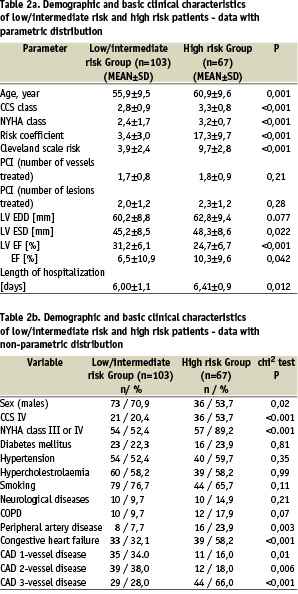
Patients were stratified based on the Cleveland surgical risk score and subsequently enrolled into two groups: group 1 - low and intermediate risk patients (estimated risk of death < 6%) and group 2 - high risk patients (estimated risk of death (6%). The characteristics of both groups is shown in Table 2.
Technique of percutaneous revascularization and definition of procedural success
Prophylactic intra-aortic balloon countrepulsation (IABP) and percutaneous cardiopulmonary support (CPS) were used only in the subgroup of high risk patients with complex stenotic lesions in coronary vessels supplying >50% of viable myocardium coexisting with significantly depressed left ventricular function (LVEF <25%). Therapy with acetylsalicylic acid (ASA) and a thienopyridine (clopidogrel or ticlopidine) was initiated at least 2 days before the procedure. The intravenous GP IIb/IIIa blocker was used at the operator’s discretion only in procedures performed in patients with complex coronary lesions and unstable angina. The unfractionated heparin (UFH) was used during the procedure to maintain the activated clotting time (ACT) at 200-300s in patients treated with the blocker and 300-400s in patients not receiving it. In all eligible cases primary stenting of the target vessel was performed. In all other cases predilatation with a balloon with smaller diameter than the reference segment of the target vessel was done. The result was regarded as angiographic success if at least one of the coronary vessels supplying a substantial area of viable myocardium or being the culprit vessel associated with anginal symptoms (CCS III-IV) was dilated with final TIMI 3 flow and residual stenosis <50%. Clinical efficiency was defined as the procedure associated to angiographic success without subsequent myocardial infarction and/or death during the index hospitalization.
Early and late outcomes - major adverse events
The following major adverse events (MAE) were analyzed separately up-to and after 30 days from index procedure as primary outcome measures:
– myocardial infarction defined as increase of CPK-MB activity exceeding 3 times value of the upper normal limit
– heart failure defined as pulmonary oedema or sustained hypotension (<90/60 mmHg) requiring mechanical cardiopulmonary support (IABP) or vasopressor infusion
– severe arrythmia defined as sustained ventricular tachycardia or ventricular fibrillation
– new-onset renal failure (increase of creatinine levels >1,8mg%) or worsening of existing chronic renal failure with the increase of creatinine levels by more than 1 mg/dl and/or requiring haemodialysis in patients not previously dialysed
– gastrointestinal bleeding associated with the decrease of hematocrit < 30% and/or need for blood transfusion
– ischemic stroke or intracerebral bleeding
– puncture-site complications requiring blood transfusion and/or surgical intervention
– acute/subacute in-stent thrombosis
– repeat-PCI or CABG
– death
The following events were recorded during follow-up after 12 months post PCI in addition to above-defined MAE:
– increased severity of angina or decompensation of heart failure requiring hospitalization
– angiographically documented culprit vesssel restenosis
– atrial fibrillation requiring cardioversion
The study end-points were:
– left ventricular ejection fraction (LVEF), left ventricular end diastolic amd end systolic diameters (LVEDD, LVESD) assessed echocardiographically in 4-chamber view one year after the procedure
– angina severity (CCS class) and heart failure functional NYHA class one year after the procedure
– angiographic and clinical success
– death or other MAE after 30 days and 1 year after procedure.
Echocardiography and treadmill stress-test
Echocardiographic evaluations of LVEF were carried out by an experienced independent operator blinded to the treatment assignment. LVEF was assessed according to the recommendations of the American Society of Echocardiography with a 16-segment model. Echogardiographic studies were carried out in certified echocardiographic core lab. The treadmill stress-test was performed according to ACC/AHA guidelines.
Safety and legal issues
The study protocol was approved by the institutional Ethics Committee and all patients gave a written informed consent to participate in the study.
The procedures were performed in Upper Silesian Heart Center where PCI procedures are carried out by experienced interventional cardiology team in a high-volume cath-lab facility (over 4000 PCI per year) with cardiac surgery back-up on site. All included cases were discussed in details with experienced cardiac surgery team on routine basis to assess the risk and benefit of surgical revascularization.
This work has been supported by the grant of Polish Ministry of Science and Informatics No. 6PO5B13221.
Statistical analysis
The data with parametric distribution were expressed as mean and standard deviation and nonparametric data as absolute number and percentage. The parametric variables in both groups were compared using unpaired Student’s t-test. The comparisons within one group were done using the Wilcoxon test and U Mann-Whitney test was used for comparisons between the groups. Survival curves were drawn using Kaplan-Meier analysis. Cox-Mantel test, F-Cox and/or Gehan tests were used for comparison of the survival curves between the treatment arms. For the comparisons of the observed and calculated survival based on the Cleveland risk 95% confidence intervals (95%CI) were calculated.
Results
Technique and procedural success of PCI
The mean of 1.73±0.84 vessels and 2.13±1.21 stenoses were treated in one procedure. Coronary stents were used in 95% of stenoses treated and mean of 1.71±0,72 stents per procedure were used. Only 5% of stented segments were covered with DES. Localization of target stenoses and frequency of stent deployment in treated vessels is shown in Table 3. Complete revascularization was achieved in 38.8% of cases.
Prophylactic IABP was used in 34 cases (20%) and mechanical cardiopulmonary support (CPS) in 6 cases (3,5%). IABP was inserted during procedure in 2 cases due to developing low output heart failure. Platelet IIb/IIIa receptor blocker (eptifibatide or abciximab) was given before starting the procedure in 12 patients (7%) and additionally in 5 patients (3%) during intervention as a bail-out treatment. Angiographic success was achieved in 98,8% and clinical success in 97,6% of cases. There was no procedure related death within 24 hours after the PCI.
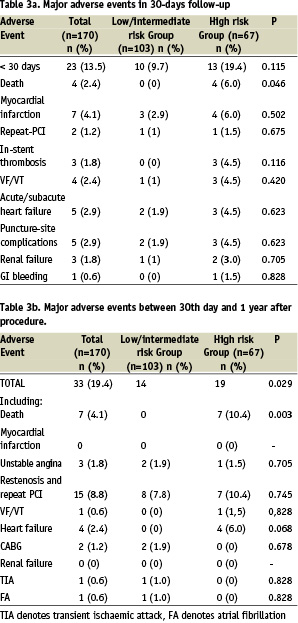
Major adverse events and long term survival
Table 3 shows the incidence of MAE in 30-days follow-up and Table 4 shows the results of 1 year follow-up. The Kaplan-Meier’s survival and survival free of MAE curves in whole study population are shown in Figure 1. Survival rate at 30-days was 97% and 12-month survival was 94,4%. The comparison of incidence of MAE between the low/intermediate risk group and high risk group revealed statistically significant lower number of MAE in group with low/intermediate risk according to the Cleveland scale (Figures 2 and 3). In low/intermediate risk group there were no deaths, both in short term and long term follow-up.
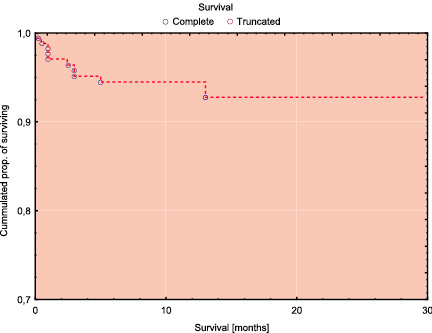
Figure 1a. Kaplan-Meier survival curves for the whole study population.
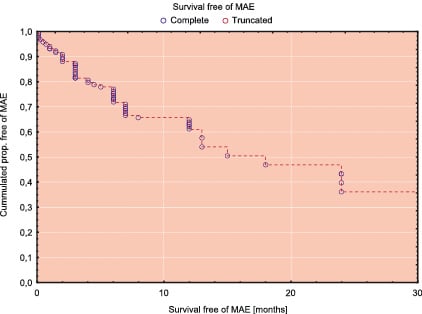
Figure 1b. Kaplan-Meier survival free of MAE for the whole study population
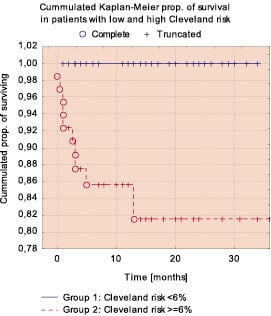
Figure 2a. Comparison of Kaplan-Meier survival curves in low/intermediate and high risk groups.
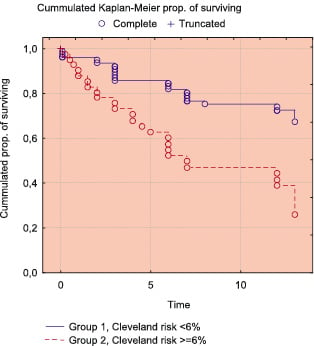
Figure 2b. Comparison of Kaplan-Meier survival free of MAE in low/intermediate and high risk groups (Cox-Mantel test: p=0.00007; Gehen-Wilcoxon test: p=0.00067).
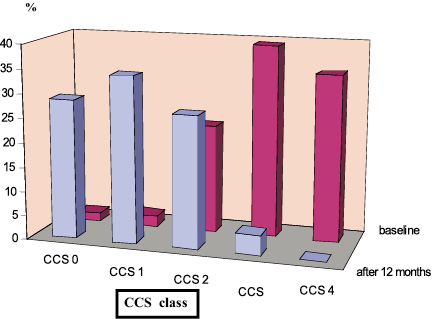
Figure 3. Clinical follow-up of PCI patients (n=170). Comparison of angina severity (CCS class) baseline and after 12 months.
Early, 30 day survival was 96.9% (95%CI: 94.3-99.5), which was better than predicted 91,2% (95%CI:89.7-92,6%) recent survival rate after CABG. Similarly, 100% survival in low/intermadate risk group and 95% (95%CI: 96,1-97,3%) in high risk group competed favorably with calculated outcome based on historic CABG model : 95% (95%CI:96,1-97,3%) and 82,7% (995%CI: 80,4-85,1) respectively.
The observed long-term survival was not inferior to predicted early outcomes after CABG.
Angina severity and NYHA functional class
Long term follow-up of the whole study population showed a significantly lower severity of angina symptoms according to CCS scale (Wilcoxon test: Z=9,83, p<0.00001) and functional improvement in NYHA class (Wilcoxon test: Z=8,99, p<0,00001) (Figures 4 and 5). Subgroup analysis revealed that patients from high risk group had significantly more severe anginal symptoms and worse functional NYHA class significantly at baseline (U Mann-Whitney test for both parameters p<0.0001). In both groups a significant improvement of both angina severity and NYHA class in 1-year follow-up was observed (Wilcoxon test for both groups p<0,00001). After 12 months there were no significant differences between the groups as to angina severity and NYHA class (U Mann Whitney test, p=0,406 and 0,094, respectively).
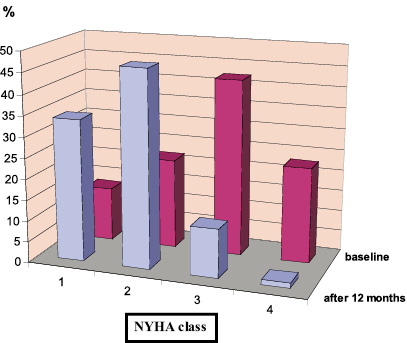
Figure 4. Clinical follow-up of PCI patients (n=170). Comparison of heart failure functional status (NYHA class) baseline and after 12 months.
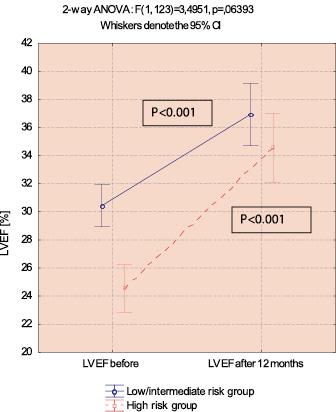
Figure 5. LVEF improvement in low/intermediate and high risk groups in 1 year follow-up (ANOVA and Newman-Keuls’ test).
Left ventricular function
After 12 months in surviving patients there was significant reduction of LVEDD from 61,7±8.5 to 59,4±7,8mm (p=0,026) and LVESD from 47,6±8,3 to 44,2±8,3 (p=0,0026).
The ANOVA test did not show any significant difference between the groups for those changes of LV diameters.
There was a significant increase of LVEF from mean 27,8±6,9 to 35,9±9,4% for the whole surviving population (p=0.000001). The mean increase of LVEF was 8,16±10,5% and was significantly lower in low/intermediate risk group in comparison to high risk group (6.5±10,9 vs. 10,3±9,6%, p=0,0423). The ANOVA test revealed a trend towards better improvement of LVEF in high risk group, however in both groups the increase of LVEF was significant.
Discussion
Our results suggest that percutaneous coronary intervention in postinfarction patients with markedly reduced left ventricular ejection fraction and documented substantial area of viable myocardium is associated with a significant increase of LVEF and favorable clinical outcome as shown by functional improvement in CCS and NYHA class. Moreover, the early postprocedural risk of death in PCI group was significantly lower as compared to calculated risk for CABG in the same patients.
The Cleveland risk scale6 for surgical patients was used in this study and survival analysis showed that it also was useful for risk stratification in patients with significantly reduced LVEF undergoing PCI. In REHEAT patients with calculated early risk <6% according to Cleveland scale there were no deaths both in short and long term follow-up. Conversely, in patients with predicted high early surgical risk - the risk of death and MAE related to PCI was significantly higher than in low risk group. The beneficial outcomes of percutaneous revascularization was observed in both high and low/intermediate risk patients, however the LVEF improvement was more marked in high risk subgroup.
According to present ESC and ACC/AHA guidelines, patients with low left ventricular ejection fraction and significant coronary stenoses should be referred to surgical revascularization7. Our data however suggest that PCI is safe, feasible and associated with favorable outcomes in terms of low risk of MAE and thus can be an alternative approach to myocardial revascularization in such patients. It seems that in experienced invasive facilities this approach may be better both in low/intermediate and high risk patients in comparison to CABG.
So far the AWESOME Randomized Trial and Registry was the only study comparing PCI with stent implantation and CABG in patients with high surgical risk and ischemic cardiomyopathy with LVEF<35%8-9. The study showed similar early and long term survival after percutaneous and surgical revascularization in this selected cohort of patients. The other studies10-17 pertain to patients with normal or near-normal LVEF (mean > 50%) and show comparable early outcomes in PCI and CABG groups. However the incidence of major adverse cardiovascular events - primarily restenosis and repeat-PCI - is higher in long-term follow-up of PCI patients. In REHEAT population the incidence of MAE might have been affected by coexisting heart failure and other extracardiac diseases which were frequent in high-risk patients. Despite this, both long term survival and survival free of MAE was better than in other clinical trials investigating medical therapy in postinfarction patients with compromised left ventricular function and comparable LVEF3,18-19.
The early and long term outcomes in patients with ischaemic cardiomyopathy undergoing CABG may be better if the GP IIbIIIa blockers and drug-eluting stents (DES) were used more frequently, especially in high-risk patients5,20. ARTS II study results showed that DES implantation in patients with multivessel coronary stenoses is associated with long term clinical outcomes comparable to that of surgical revascularization20. In patients with low LVEF and major coexisting diseases leading to increase in surgical risk, the use of DES may additionally improve long-term outcome as well as reduce the risk of periprocedural MAE.
The relatively low use of glycoprotein IIb/IIIa inhibitors in our study was related to the fact, that this efficient, but costly treatment is not reimbursed by the second payer in our country, unless the procedure is performed in acute coronary syndrome patients.
In conclusion, our results suggest the favorable risk/benefit profile of PCI in patients with ischemic cardiomyopathy and low LVEF.
However, the randomized clinical trial comparing CABG and PCI with routine use of DES implantation and modern antiplatelet/anticoagulation therapy21 should be carried out to prove finally this concept.

