The goal of treatment of patients with acute myocardial infarction (AMI) is to achieve optimal and rapid restoration of coronary blood flow in the infarct-related vessel and to maintain the initial result at follow-up. There are two main treatment methods of re-opening an occluded artery: administrating a thrombolytic agent and primary percutaneous coronary intervention (PCI) with or without stenting. In the 1990s and the beginning of the 2000s, many randomized studies comparing these two reperfusion procedures have shown that primary PCI with routine stent implantation for AMI has a better outcome than thrombolytic therapy1,2 or balloon angioplasty3-5. A recent meta-analysis also indicated that primary PCI was more effective than thrombolytic therapy for the treatment of ST-segment elevation myocardial infarction (STEMI)6.
In the last 3 to 4 years, drug-eluting stents (DES), either sirolimus-eluting stents (SES) or paclitaxel-eluting stents (PES), have been used in various clinical settings and have revolutionized the interventional cardiology practice by reducing restenosis and revascularization rates in comparison to bare metal stents (BMS). Two pivotal trials using DES in relative high risk patients with complex lesions reported single-digit restenosis rates and a lower incidence of revascularization than BMS7,8. These trials, however, only included elective procedures. Despite the lack of sufficient evidence, the experience in stable patients was extrapolated to patients with unstable angina pectoris, non ST-segment elevation acute coronary syndrome (NSTE-ACS) and ST-segment elevation acute coronary syndrome (STE-ACS); thereby the large number of DES currently used in urgent procedures.
Previous studies suggested that sirolimus could alter and decrease endothelial function in vitro9 and in humans10, enhance agonist-induced platelet aggregation11 and tissue factor expression12, and delay vascular healing13-15. Moreover, a hypersensitivity reaction to the polymer coating of SES was observed16-18. As for paclitaxel, a recent study suggested PES also induced a hypersensitivity reaction18. These features of DES can increase the risk of thrombotic complications and have led to prolonged antiplatelet treatment and cautious use of DES in the acute phase of unstable angina and myocardial infarction. Indeed, discontinuation of antiplatelet drugs was a strong independent factor of DES thrombosis19-21 and a recent publication reported on patients treated with PES for AMI who presented with late stent thrombosis after cessation of antiplatelet drugs22.
So far, only 7 reports about DES implantation in AMI patients were published (see Table).
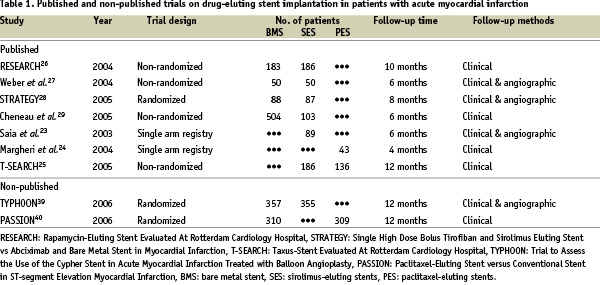
One study evaluated clinical and angiographic outcomes of SES implantation in a consecutive series of 89 patients23. Another study analysed short-term clinical outcomes of consecutive 43 patients treated with PES implantation24. The latest report made a comparison of the efficacy between PES and SES25. The other 4 studies26-29 assessed the advantage of SES over BMS. We compiled the results of these 4 studies and performed a meta-analysis, as there were no published trial comparing PES with BMS in the clinical setting of AMI. The range of clinical follow-up time varied between 6 and 10 months.
It should be mentioned that the study design and methodology of the four studies included in the present meta-analysis were variable, and all of them were conducted in one single centre. The STRATEGY (Single High Dose Bolus Tirofiban and Sirolimus Eluting Stent vs Abciximab and Bare Metal Stent in Myocardial Infarction) trial28, was the only prospective, single blind, randomized controlled study presenting 8 months of follow-up. The Rotterdam study26, as a part of the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) registry, and the German study27 adopted a similar methodology. Patients treated with SES were prospectively enrolled and compared to control patients treated with BMS in the immediate preceding period before the introduction of SES. Enrolled patients were followed for 10 and 6 months, respectively. The last study was a retrospective analysis29. SES implanted patients were compared to patients treated with BMS in the preceding 5-year period before the approval of SES, and followed up to 6 months. The robustness of each study is variable, so that some limitations may be created in the result of our tentative meta-analysis, which –as we should point out– does not collect individual data of enrolled patients in the 4 studies.
The result of the meta-analysis is shown in the accompanying figure 1.
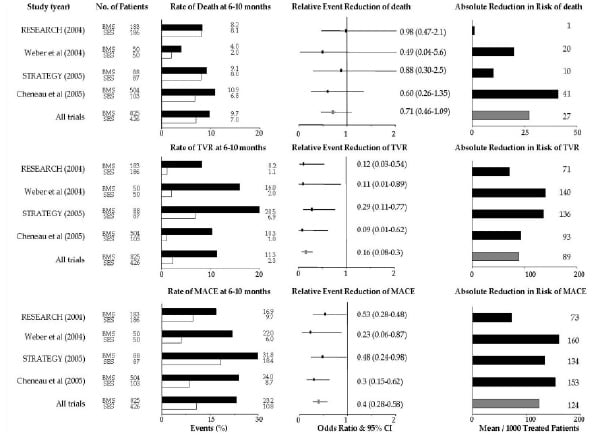
Figure 1. Rates and the relative and absolute reduction in the risk of death, TVR and MACE among patients treated with SES, as compared with those treated with BMS, at six to ten months. Open boxes represent patients treated with SES. Shaded boxes represent patients treated with BMS. Off-white boxes represent overall results. BMS: bare metal stents, SES: sirolimus-eluting stents, RESEARCH: Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital, STRATEGY: Single High Dose Bolus Tirofiban and Sirolimus Eluting Stent vs Abciximab and Bare Metal Stent in Myocardial Infarction, TVR: target vessel revascularization, MACE: major adverse cardiac events, CI: confidential intervals.
Overall, SES is associated with a 28% relative reduction in all-cause deaths, an 80% relative reduction in target vessel revascularization (TVR), and a 53% relative reduction in major adverse cardiac events (MACE), which include all-cause mortality, non-fatal myocardial infarction, and TVR (we could not analyse the non-fatal myocardial infarction rates because one study26 did not state the incidence of this complication separately). These results provide for a better outcome after SES utilization in patients with AMI. Noteworthy, the rate of TVR is similar to values reported in randomized trials of elective SES use.
At follow-up, the incidence of death in patients treated with SES was comparable in 3 studies with the exception of the German study; it was approximately 7% to 8%, which seemed similar to the one observed in the control arms (approximately 8% to 10%). As the German study included patients with not only STE-ACS but also NSTE-ACS and excluded patients presenting cardiogenic shock, this might be the explanation that its mortality rate is lower than in the other studies. In the pooled data, short-term (30 days) mortality rate was 3.8% in the SES arm and 3.4% in the BMS arm. Moreover, no angiographic stent thrombosis, including acute, subacute and late thrombosis, was seen in patients treated with SES. In the BMS arm, the subacute thrombosis rate was 1.1%, which is comparable with the incidence of BMS thrombosis in the treatment of patients for stable coronary lesions30. No late stent thrombosis was observed in the BMS arm. The fact that patients with DES tended to take antiplatelet therapy during longer period than control patients might affect these results. However, SES is likely to be as safe as BMS and does not seem to increase the thrombogenic complications in the clinical setting of AMI at least in both short- and medium-term.
In each study, the medium-term TVR rate in the SES arm was remarkably lower than in the BMS arm. The TVR rate of the pooled data in patients treated with SES was only 2.3%, whereas 11.3% of patients treated with BMS underwent revascularization and this rate was close to the result of previous studies1-3. In the STRATEGY trial, the TVR rates in both stent arms were higher than those observed in the other 3 non-randomized trials. The reason for this discrepancy is not readily apparent. The lesions of patients who were enrolled in the STRATEGY trial might have been more complex than those in the other trials. It might also reflect the fact that different glycoprotein IIb/IIIa inhibitors were used in each arm; abciximab was administrated in the BMS arm and single high dose bolus tirofiban (25 µg/kg) in the SES arm.
Among the 4 studies, angiographic assessment was systematically performed for enrolled patients in the STRATEGY trial and the German study. Only the first 89 consecutive patients in the RESEARCH registry also underwent angiographic analysis23. The late lumen loss in SES arms ranged from –0.22 mm to 0.12 mm and the binary restenosis rate varied from 0% to 11%. Negligible, or very small late lumen loss is associated with a lower incidence of angiographic restenosis as well as a reduced need for TVR, which was well described in many previous trials investigating patients with stable coronary disease31-34. Such an angiographic analysis indicated that SES retained its ability of inhibiting neointimal formation even in the clinical setting of AMI. Consequently, the medium-term MACE rate in the SES arm was significantly lower in comparison with the BMS arm (10.8 % vs 23.2%, respectively; p < 0.001). Low TVR rates accounted for lower MACE rates in patients treated with SES.
Recent head-to-head (SES vs PES) trials indicated that SES was significantly better at reducing neointimal hyperplasia and had a slightly advantage over PES in clinical outcomes, such as target lesion revascularization (TLR) and TVR35-37. Similarly, the study of Hofma et al.25, which was the only study comparing the efficacy of SES and PES in the clinical setting of AMI and published as a subanalysis of the Taxus-Stent Evaluated At Rotterdam Cardiology Hospital (T-SEARCH) registry38, showed a higher rate of TLR, TVR and MACE in the PES arm than in the SES arm. In the PES arm, 2.9% of all patients experienced stent thrombosis, whereas in the present meta-analysis we did not find any stent thrombosis in the pooled SES arms as mentioned previously. Different drug-release kinetics and mechanism of neointimal inhibition between SES and PES might have contributed to the observed difference in their performance. At the present time, we cannot conclude that PES is inferior to SES in patients with AMI. Further investigations are needed to identify which DES is more effective in this clinical setting.
In conclusion, our analysis suggests that SES is a safe and effective device for the prevention of restenosis in the clinical setting of AMI. As a result, the use of SES leads to low TVR and MACE rates, and seems to be superior to BMS even in this subset of patients. SES appears to be an attractive therapeutic approach for patients admitted with AMI. However, the number of enrolled patients in each study was too small so that they are underpowered to assess definitely the effect of SES on the rate of TVR or MACE. As for PES utilization in AMI, only a few trials were published. Much more information are needed to confirm whether PES implantation in patients with AMI is safe and feasible compared to BMS or SES. To establish the safety and advantage of both DES in AMI, larger randomized trials are imperative.
At the American College of Cardiology 2006 Scientific Sessions, two multi-centre and randomized trials about DES implantation in the clinical setting of AMI were presented: the TYPHOON39 (Trial to Assess the Use of Cypher stent in Acute Myocardial Infarction treated with Angioplasty) trial and the PASSION40 (Paclitaxel-Eluting Stent versus Conventional Stent for ST-segment Elevation Myocardial Infarction) trial. The TYPHOON trial included 712 patients and assessed the effectiveness and safety of SES as compared to BMS at 1 year. The TVR rate in patients treated with SES was significantly lower than those who received BMS (5.6% vs 13.4%, p < 0.001). The MACE rate was also lower in the SES group (5.9% vs 14.6% in the BMS group, p < 0.001). These clinical outcomes confirm the result of our meta-analysis, if not quantitatively at least qualitatively. The PASSION trial, which enrolled 619 patients, was the first trial comparing PES with BMS in the clinical setting of AMI. This trial failed to find an advantage of PES over BMS in terms of MACE (8.7% and 12.6%, p = 0.12) and TLR (6.2% and 7.4%, p = 0.23) at 1 year. These findings are at variance with the results of the TYPHOON trial. We will have to wait for the results of the HORIZONS trial, in which 3400 patients will be randomized and the superiority of PES over BMS will be investigated.

