Abstract
Aims: Drug-eluting stents are being increasingly used for the treatment of de novo coronary lesions. This trial compared the performance of tacrolimus-eluting stents (TES) with that of equivalent carbon-coated stents (CCS).
Methods and results: The JUPITER II trial enrolled 332 patients (mean age = 64 years, 75% men) with de novo coronary stenoses. Patients were randomly assigned to TES versus CCS and underwent repeat coronary angiography at 6 months. The primary study endpoint was in-stent and in-segment late lumen loss (LLL). The main secondary endpoints included binary restenosis, and rates of major adverse clinical events (MACE), including death, myocardial infarction, target lesion revascularisation and stent thrombosis at 1, 6, and 12 months of follow-up. The procedure was successful in 99.4% of patients in both groups. There was no procedural death, 1 cardiac death at 2 months, and 1 acute and 2 subacute CCS thromboses. Angiographic follow-ups were available in 94.4% and 95.1% of patients assigned to TES and CCS, respectively. The mean in-segment and in-stent LLL was 0.42±0.46 mm and 0.65±0.47 mm, respectively, in the TES, versus 0.48±0.52 mm and 0.66±0.53 mm, respectively, in the CCS group (ns). The 12-month cumulative MACE rate was 19.5% in the CCS versus 16.1% in the TES group (ns).
Conclusions: Both stents showed high safety, with no stent thrombosis in the TES group. No difference was observed in 6-month angiographic results and 12-month MACE rates between TES and CCS.
Introduction
The success of percutaneous myocardial revascularization has undergone a profound evolution since its inception, nearly 30 years ago, when Grüntzig made his first attempts at dilating coronary stenotic lesions with custom-made balloons.1 The introduction of tubular metal stents to scaffold the vessel represented a major advance in the fight against unacceptably high rates of restenosis after angioplasty alone. In addition, coating of the stents with substances that inhibit the proliferation of neointimal tissue, has further enhanced the performance of percutaneous coronary interventions in most patients with various clinical presentations, including multivessel disease,2,3 complex and very long coronary lesions,4-6 and acute myocardial infarction (MI).7
While, in the long term, and compared with bare metal stents, drug-eluting stents are effective in reducing the incidence of major adverse cardiovascular events (MACE), patients who undergo implantation of multiple and overlapping stents,8,9 patients with very small vessels,10,11 saphenous vein grafts,12 or insulin-dependent diabetes,13 continue to suffer relatively high restenosis rates. Furthermore, there is a lingering concern that drug-eluting stents might be associated with higher rates of late thrombosis, which threatens the long-term health and survival of patients who have undergone uneventful implantation procedures,14,15 for which prolonged dual antiplatelet therapy has been recommended. While the precise mechanism of late thrombosis has not been clarified, the durable polymer coating used to deliver the drug is under scrutiny. Therefore, the introduction of new substances able to inhibit neointimal proliferation with no increase in stent thrombosis, and of safer delivery methods with no polymer, are important endeavours.
Tacrolimus (FK506), a lipophilic macrolide immunosuppressant, lowers the incidence and severity of organ rejection after transplantation.16 In a porcine model of overstretched coronary stent, the stent-based delivery of tacrolimus inhibited the formation of neointimal tissue, an effect which was associated with a decreased inflammation around the struts.17 In addition, in 2 groups of non-atherosclerotic minipigs, the implantation of tacrolimus-eluting (TES) was associated with a 61% reduction in neointimal thickness compared with the implantation of equivalent carbon-coated (CCS) Carbostents, (unpublished observations presented orally by Antonio Bartorelli, MD at the 2004 Transcatheter Cardiovascular Therapeutics meetings, Washington, DC). Furthermore, in human cells, tacrolimus was found to have distinct antiproliferative properties toward smooth muscle cells, without prominent antiproliferative effects toward endothelial cells.18 In a “First-In-Man” (FIM) study (unpublished observations presented orally by Antonio Bartorelli, MD at the 2004 Transcatheter Cardiovascular Therapeutics meetings) the implantation of TES was associated with a 3.8% binary restenosis rate at the 6-month angiographic follow-up in non-diabetic patients, and 16.9% binary restenosis rate in diabetics. No stent thrombosis was observed during a 12-month clinical follow-up. Because of the high restenosis rate observed in diabetics in this “First-In-Man” study, the dose of tacrolimus on TES was increased from 1.5 µg/mm2 to 2.3 µg/mm2.
This multicentre European clinical trial was conducted to compare the safety and effectiveness of direct stenting with Janus™ TES (Sorin Biomedica Cardio s.r.l., Saluggia, Italy), versus the mechanical platform with no drug eluting capability from which the Janus stent was derived (Tecnic™ CCS coronary Carbostents) in the treatment of de novo stenoses in native coronary arteries of patients presenting with angina pectoris or silent myocardial ischaemia.
Patient population and methods
Between March, 2004 and December, 2004, candidates for percutaneous myocardial revascularisation presenting with stable or unstable angina, or silent ischaemia at 16 European medical centres (Appendix), were invited to participate in a randomised trial to compare the performance of Janus™ with that of Tecnic™ coronary stents. The tubular structure of both stents, made of medical grade stainless steel, is coated with a high-density carbon film (Carbofilm™). In addition, the Janus™ stent is loaded with a 2.3 µg dose of tacrolimus contained in multiple reservoirs located on the stent’s outer surface, limiting the release of the drug towards the vessel wall.
This study was reviewed and approved by the Ethics Committees of each participating institution, and informed, written consent was obtained from all patients before their random assignment. They were eligible for randomisation if they fulfilled the following clinical criteria: a) age >18 and <85 years, b) able and willing to comply with the follow-up schedule and procedures, c) candidate for percutaneous myocardial revascularisation for stable or unstable angina as defined by the Canadian Cardiology Society and by Braunwald,19,20 or with silent ischaemia. Furthermore, the following angiographic criteria had to be fulfilled: 1) >50% and >100% (by visual estimate) de novo coronary lesions in up to 2 native vessels between 2.7 mm and 4.0 mm in reference diameter by visual estimate, suitable for treatment by direct stenting; b) >20 mm target lesions that could be covered with a study stent 5-mm longer than the target lesion.
Patients were excluded from randomisation if they a) had sustained a MI within the last week (unless a Q-wave MI, and CK and CK-MB enzyme concentrations has returned to normal), b) were pregnant, c) had <1 year life expectancy from a concurrent disorder, d) had a serum creatinine concentration >2 mg/dl, or elevated liver enzymes, e) suffered from a bleeding disorder precluding treatment with clopidogrel or aspirin, f) had a ≤40% left ventricular (LV) ejection fraction or prominent segmental LV wall abnormalities, g) had a target lesion <10 mm away from a previously implanted stent in the same vessel, >2 treatable lesions in the target vessel, or target lesion in the left main coronary trunk, h) presented with a large thrombus, tortuosities, calcifications, ostial or bifurcation location, >50% unprotected left main artery stenosis or other findings precluding the safe performance of angioplasty or stenting, i) were intolerant of aspirin, ticlopidine, clopidogrel or hyper-sensitive to tacrolimus, k) had an abnormal blood cells count, l) participated in another research protocol.
Randomisation was performed in a 1:1 ratio, in a double-blind fashion, by opening by the investigator of closed envelopes containing a number corresponding to a treatment group assignment.
Baseline evaluation and follow-up
At the time of study entry, and at all subsequent scheduled or unscheduled visits, the patients underwent a detailed evaluation, including demographic and laboratory profiles, scoring of anginal status and myocardial ischaemia, measurements of hepatic and cardiac enzymes, and recording of a 12-lead electrocardiogram. Stress tests were performed when considered indicated by the investigator. Clinical follow-up was performed at 1, 6, and 12 months. In addition, at the 6-month visit, the patients underwent protocol-mandated coronary angiography with quantitative measurements.
Baseline and follow-up coronary angiography
Patients underwent cardiac catheterisation according to procedures routinely adopted at the participating medical centres, chosen for their known expertise in interventional cardiology and coronary stent implantation, and positive track record of participation in multicentre clinical studies. Prior to the stenting procedure, diagnostic angiography was performed in at least 2 orthogonal projections, at a minimum speed of 12.5 frames/sec, with no overlapping of branches and minimal foreshortening of the target lesion. Angiography was repeated in the same projections immediately after the procedure and at 6-month follow-up, to verify the patency of the target vessel, and monitor the development of in-stent or in-segment restenosis. Coronary angiography was also repeated upon the occurrence of adverse clinical events, as clinically indicated. All scheduled and unscheduled angiograms were acquired after intracoronary injection of 100-300 µg of nitroglycerin or 1-3 mg of isosorbide dinitrate. All procedures, recorded on CD-ROM, were forwarded to 2 independent core laboratories (Bio Imaging, Heart Core B.V., Leiden, The Netherlands, and Cardialysis, Rotterdam, The Netherlands) for quantitative coronary angiography (QCA). The results presented in this article are those that were reported by Cardialysis, which were similar to those reported by Bio Imaging. The guiding catheter was used as calibration device. A computer-assisted 5.1 QCA system (CAAS II, Pie Medical Imaging bv, Maastricht, The Netherlands) was used for analysis, with the technician blind to the randomisation code. After stenting and at follow-up, the measurements of the segment of interest were divided into stented segment, demarcated by proximal and distal platinum markers, and 5-mm proximal and distal margins on each side.
Stent implantation procedure
Percutaneous access was obtained according to the procedures routinely adopted at each individual participating centre. After the introduction of 6F, 7F or 8F guiding catheters into the access site, intravenous heparin was administered to reach and maintain an activated clotting time (ACT) >300 sec (<250 sec, if abciximab was administered concomitantly). After verification that the target lesion satisfied all angiographic inclusion criteria, the randomisation process was completed, a 0.014” guide wire was used to cross the lesion and advance the assigned stent to the target lesion without predilatation. The stent was selected to exceed the target lesion length by at least 2.5 mm on each side. Stents between 3.0 and 3.5 mm in diameter, and between 12.0 and 25.0 mm in length, were available for this study. A minimal pressure of 12 atm was used for stent deployment. The implant procedure was completed when a <20% residual stenosis with TIMI flow III had been achieved. When this endpoint was not reached, the stent was post-dilated with the same delivery balloon, or with a shorter, non-compliant balloon, to limit the risk of dissection. Coronary dissections present at the end of the procedure were treated with additional stents from the same randomly assigned group.
Antiplatelet therapy
Clopidogrel, 300 mg, p.o., was administered on the day of the procedure as a loading dose, unless the patient had been pre-treated with clopidogrel. Aspirin 75-100 mg, p.o was administered before the procedure. Patients who had not previously been on long-term therapy, received a 500 mg intravenous dose of soluble aspirin before the procedure. The administration of glycoprotein IIb/IIIa receptor antagonist was left to the investigators’ discretion.
After completion of the index procedure, patients were placed on a regimen of clopidogrel, 75 mg/day, for at least 2 months, and aspirin, >75 mg/day, indefinitely.
Definitions and study endpoints
Late lumen loss (LLL) was defined as the difference between minimum luminal diameter (MLD) at the end of the index procedure, compared with the 6-month follow-up, measured by QCA, in each study group. MACE was defined as death, MI, target lesion revascularisation (TLR), target vessel revascularisation (TVR) and stent thrombosis. Myocardial infarction was diagnosed on the basis of new pathological Q wave, or an increase in creatine kinase enzyme concentration to twice the upper limit of normal associated with an elevated creatine kinase-MB concentration. Stent thrombosis was defined as the angiographic documentation of a complete or subtotal occlusion (TIMI flow 0 or 1) of an artery previously successfully treated (TIMI flow 2-3 immediately after the procedure and <50% residual %DS), or angiographic documentation of a flow limiting thrombus (TIMI flow 0 or 1) of an artery previously successfully treated. Thrombosis was defined as acute if it occurred after the removal of the guiding catheter, up to 24 h after the index procedure, subacute between 25 h and 30 days, and late past 30 days, after the index procedure.
The primary endpoint of the trial was in-stent and in-segment LLL. The secondary endpoints were 1) binary angiographic restenosis at 6-month angiographic follow-up, 2) MACE at discharge, and at 1, 6, 12 and 24 months of follow-up, 3) rates of TLR at 6, 12 and 24 months of follow-up, 4) in-stent and peri-stent MLD ascertained by QCA immediately after the procedure and at 6-month follow-up visit, 5) in-stent and peri-stent percent diameter stenosis (%DS) assessed by QCA immediately after the procedure and at the 6-month angiographic follow-up, 6) incidence of stent thrombosis at discharge, and at 1, 6, and 12 months of follow-up, 7) incidence of bail-out procedures for dissections after direct stenting, and 8) incidence of predilation procedures related to unsuccessful direct stenting.
Data collection, management and monitoring
Data were collected on case report forms provided by Sorin Biomedica Cardio, the study sponsor, who was also responsible for the monitoring of the trial. The consistency of the data recorded by the clinical sites was verified by an independent clinical research organisation (Clinimetrics, Omnicare, Berks, UK). Discrepancies identified through the data management review process were returned to the enrolling centre for resolution. All data were entered into a clinical electronic database maintained by the Sponsor.
Clinical Event Committee. An independent committee (Appendix) classified the adverse clinical events and adjudicated whether they were related to the investigational device. This committee also monitored the incidence of adverse events, with a view to verify the consistency and accuracy of all adverse events reported.
Sample size calculations
The patient sample size was calculated on the basis of the study primary endpoint, in-segment LLL at 6 months, assuming a 0.25-mm reduction in LLL in lesions treated with the Janus Carbostent compared with lesions treated with Tecnic. Recent clinical observations with the Tecnic stent, using the direct stenting technique, had shown a 0.66±0.87 mm mean LLL at 6 months (unpublished sub-analysis of the Phantom IV trial21). The sample size was calculated using the calculation program Sample Power 2 (SPSS Inc. Chicago, IL), using the t-test method for 2 independent groups with common variance. A minimum sample size of 151 patients in each group was calculated to detect a 0.25 mm difference in 6-month LLL between the 2 stents with an 80% power, assuming a common standard deviation of 0.87. The α parameter was set at 0.05 and the test was one-tailed, since it was expected that the study DES effect would be expressed positively with respect to the control stent. Further assuming a 10% “drop-out” rate (withdrawn, lost to follow-up and death) an enrolment of 165 patients per treatment arm was calculated to guarantee an 80% power. Finally, in order to collect statistically balanced and representative data, each centre was expected to enrol at least 10, and no more than 50 patients.
Statistical analyses
The methodology used in this study was based on standard statistical methods commonly used in the analyses of clinical events. Continuous variables are presented and were evaluated as means and standard deviation (SD). Discrete variables (e.g.: sex, risk factors, aetiology, etc.) are presented and were evaluated using frequency distributions. Comparisons between classes of continuous variable were made using Student’s t-test or analysis of variance applied in all its combinations. Comparisons among classes of discrete variable were examined using the χ2 test or, in case of scores, the Wilcoxon or similar test. The Cochran-Mantel-Haenszel test was used to examine differences among study centres (categorical variables analysis). The incidence of adverse clinical events was calculated using actuarial or the Kaplan-Meier methods. The probability of not incurring a determined adverse event was graphically examined in relationship to the follow-up period. These event analysis methods allow the detection of statistically significant differences between patient sub-groups, through the log-rank test. Statistical significance was considered reached for probability values (p) <0.05. All data were analysed with the SAS software, version 8.2 (SAS Institute Inc. Cary, NC)
Results
The trial enrolled 332 patients who fulfilled its inclusion and exclusion criteria, and were evenly assigned to each study stent. Data from all patients were analysed according the intention to treat principle. The baseline characteristics of the 2 study groups were similar (Table 1).
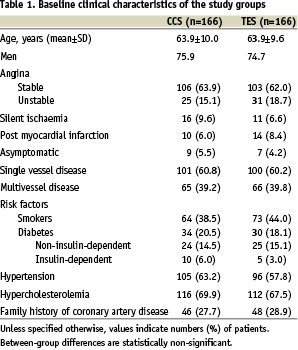
Approximately 2/3 of patients in both groups presented with stable angina and with single vessel coronary artery disease.
Procedural characteristics and outcomes
Direct stenting was attempted in 85.7% of patients in the control group, versus 75.9% of patients in the TES group. This was the only significant procedural difference between the 2 study groups (Table 2).
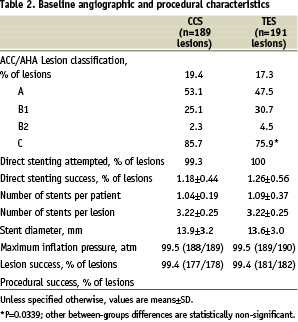
The lesion and procedure successes were nearly 100% in both groups. Three patients assigned to CCS developed acute MI and underwent emergency repeat PCI for stent thromboses. No patient died during the index hospitalisation.
Antithrombotic therapy. All patients in both groups were discharged from the hospital on a dual antithrombotic drug regimen of clopidogrel and aspirin. At 2 months of follow-up, 100% of patients in both groups, at 3 months, 62.8% of patients in the control group and 68.4% in the tacrolimus-eluting group, and at 6 months 53.3% and 52.9%, respectively, remained on dual antithrombotic therapy.
Follow-up
Angiographic data were available at 6 months for 153 patients (169 lesions) in the TES, and 156 patients (168 lesions) in the CCS group, corresponding to 94.4% and 95.1% follow-up rates, respectively. At 6 months, the mean in-segment and in-stent LLL, ascertained by QCA, was 0.42±0.46 mm and 0.65±0.47 mm, respectively, in the TES, versus 0.48±0.52 mm and 0.66±0.53 mm, respectively, in the CCS group (Table 3).
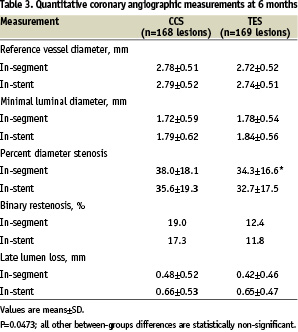
The in-segment cumulative distributions of LLL at 6 months of follow-up in each study group were similar (Figure 1).
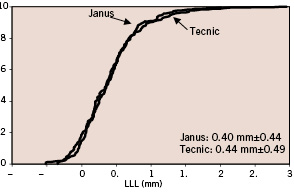
Figure 1. In-segment cumulative distribution of late lumen loss at 6 months of follow-up in each study group.
Unlike what was observed in the “First-in-Man” study, the 6-month LLL and other angiographic results among the diabetic sub-population (28 assigned to TES and 33 assigned to CCS) were not significantly different from the results obtained in the non-diabetic subgroups (data not shown).
Clinical data set were available at 6 and 12 months for 161 and 155 patients in the TES, and 163 and 159 patients in the CCS groups, respectively, corresponding to 96.9 and 98.2% follow-up rates at 6 months, and 95.1% and 95.8% at 12 months, respectively. There were 7 patients lost to follow-up in the CCS group, and 8 patients lost to follow-up and 3 deaths in the TES group. On clinical evaluation, 139 patients (89.7%) in the TES group and 143 patients (90.1%) in the CCS group were angina-free, 15 (9.7%) and 15 (9.4%), respectively, complained of stable angina, and a single patient in each group (0.6%) suffered from unstable angina. These differences were not statistically significant. The distribution of MACE at 6 and 12 months in each study group is shown in table 4.
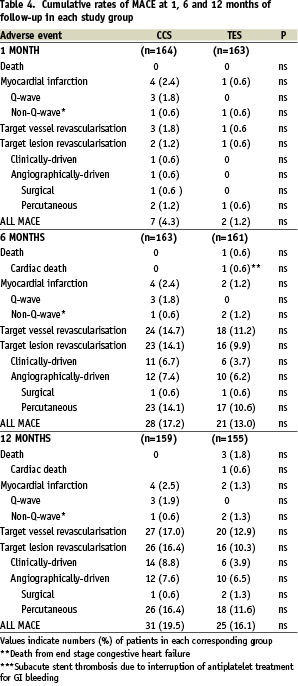
The overall, cumulative MACE rates were similarly low in both groups. No patient in either group developed subacute or late stent thrombosis.
Fatal adverse events
One patient in the TES group died 2 months after the stent implantation procedure, from intractable congestive heart failure and severe pulmonary hypertension. This death was adjudicated by the CEC as a death unrelated to the study stent. Two other patients in the TES group died during the 12-month follow-up of bronchial carcinoma and acute pyelonephritis and internal haemorrhages, respectively.
Stent thromboses and other major in-hospital cardiovascular complications
Three patients assigned to CCS developed stent thromboses, 2 of which complicated by MI, during the index hospitalisation. One of these 3 patients had developed a massive gastrointestinal haemorrhage after the diagnostic angiogram, and underwent subsequent stent implantation with clopidogrel as the only peri-procedural antithrombotic regimen. The other 2 patients developed stent thromboses shortly after removal of the guiding catheters. No patient in the TES group developed stent thrombosis. One patient in the TES group sustained an uncomplicated MI during the index hospitalisation and was treated conservatively. Finally, one patient in the TES group who had developed severe pulmonary hypertension shortly after the index PCI died of endstage heart failure at 2.5 months of follow-up.
Discussion
The Janus Carbostent consists of a stainless steel structure entirely coated with an ultra thin layer of Carbofilm™, which confers its thrombo-resistant properties. To load the drug, deep reservoirs were created on the external abluminal surface of the struts, with the aim of a selective release of the drug toward the vessel wall, without dissipation into the blood stream. No polymer is present in this drug-eluting stent. These characteristics enhance the biocompatibility and effectiveness of the stent, and increases the amounts of drug that it can carry, while decreasing, at least theoretically, its late thrombogenicity.22 In addition, the absence of polymer platform, facilitates direct stenting, since it eliminates the risk of compromising the polymer, and therefore the drug delivery, when crossing calcified lesions. It is noteworthy, however, that, besides potential advantages, the absence of polymer might also be a limitation, since it contributes to a tight control of the drug release. An unsuccessful trial (DELIVER) of a stent without polymer has already been completed, despite the use of a drug (paclitaxel) known to be effective.23
The current device is considerably different from a previously developed TES tested clinically (Jomed). The stainless steel structure of that earlier stent was coated with a nanoporous ceramic coating, used as the carrier for tacrolimus, which was eluted both towards the vessel wall and into the blood. Its clinical testing was abandoned when a higher than expected TLR rate was observed,24,25 attributed to an insufficient amount of drug loaded, as well as cracking of the ceramic coating upon expansion.
The present randomised comparison of TES versus CCS, failed to show a significant advantage of drug elution, despite the attainment of a mean LLL, in the TES group, consistent with the target value used to calculate the study sample size. This negative outcome has 3 hypothetical explanations. First, tacrolimus might not be a drug suitable to prevent stent restenosis. While the Jomed stent design was different, this represents, nevertheless, the second unsuccessful trial of a TES. Second, tacrolimus might be effective, though was delivered from the stent in insufficient amounts. However, the current stent design delivers the maximum amount of drug that can be loaded. Should this hypothesis be correct, deeper drug-containing reservoirs will need to be created. Third, the kinetics of release of tacrolimus might not have allowed the full expression of its effects against neointimal proliferation as early as 6 months. TES were designed with a first phase of drug release during which the potent anti-inflammatory action of the tacrolimus is used to prevent the early inflammatory vascular response, and a second phase aimed at controlling the proliferation of smooth muscle cells. Preclinical experiments in-vivo showed a rapid drug release in the first week, followed by a slower release in the following weeks, with approximately 50% of the drug released at 1 month.26 The results of this clinical trial suggest that a more sustained release in the second phase might be more effective. Furthermore, the unexpectedly high performance of the CCS, which resulted in the lowest 6-month LLL measured with a non-drug-eluting stent in a large controlled study, reduced the possibility of detecting a significant superiority of TES compared with CCS, and of reaching the primary study endpoint. A study comparing TES with another commercially available bare metal stent might have yielded different results. These observations also illustrate the marked variability in the performance of bare metal stents, which may depend on several stent characteristics, such as the strut thickness and the presence of carbon coating of the struts.27
The polymer platform has been suspected to promote late stent thrombosis. This concern is supported by several preclinical and clinical studies, which have shown that the polymer can cause intravascular inflammatory reactions, delayed intimal hyperplasia, persistence of fibrin and incomplete endothelialisation, pathologic changes which might all become clinically apparent only after the discontinuation of long-term antithrombotic therapy.28-31 The absence of any thrombotic complication in this trial of stents that are not polymer-based was particularly encouraging, and warrants the pursuit of further studies of tacrolimus-elution associated with the Carbofilm.
Conclusions
In this first randomised trial with direct stenting comparing TES with CCS favourable angiographic and clinical results were obtained with both types of stents. Although the 6-month LLL associated with TES was consistent with that estimated in the planning of the trial, it was not significantly less than that observed with CCS because of the latter’s higher than expected performance. In addition, the absence of stent thrombosis in the TES patient group is encouraging from a safety standpoint. Finally, while the rates of adverse clinical events were low in each study group, a trend to fewer TLR at the end of 12 months among patients randomly assigned to TES than to CCS, suggests that the angiographic observations made at 6 months may not reliably predict longer-term clinical outcomes. These unsettled issues warrant further examination in larger populations over longer periods of observations.
Appendix
The following investigators and institutions participated in the JUPITER trial:
Principal investigator: Marie-Claude Morice, MD, Institut Hospitalier Jacques Cartier, Massy, France; Co-investigators: Wim Aengevaeren, MD, University Medical Center Radboud, Nijmegen, The Netherlands; Franz Amann, MD, Herz Gefass Zentrum Zurich, Klinik im Park, Zurich, Switzerland; Didier Carrié, MD, Hospital de Rangueil Service de Cardiologie B, Toulouse, France; Alberto Cremonesi, MD, Casa di Cura Villa Maria Cecilia, Cotignola, Italy; Bernard De Bruyne, MD, William Wijns, MD, Onze-Lieve Vrouw Ziekenhuis, Aalst, Belgium; Carlo Di Mario, MD, Charles Ilsley, MD, Royal Brompton and Harefield NHS Trust, London, United Kingdom; Carlos Macaya, MD, Hospital Clinico San Carlos, Madrid, Spain; Hans-Peter Bestehorn, MD, Herz-Zentrum Bad Krozingen, Bad Krozingen, Germany; Otmar Pachinger, MD, University of Innsbruck, Innsbruck, Austria; Patrick Serruys, MD, Erasmus MC Klinische Epidemiologie Thoraxcentrum, Rotterdam, The Netherlands; Stefan Verheye, MD, A.Z. Middelheim, Antwerp, Belgium; Christophe Dubois, MD, U.Z. Gasthuisberg, Leuven, Belgium; Stefan Hoffmann, MD, Vivantes Friedrichshain/Urban, Berlin, Germany; Albrecht Hempel, MD, Stadtisches Klinikum, Dresden, Germany; Robert de Winter, MD, Academic Medical Center, Amsterdam, The Netherlands.
Clinical Events Committee
Antonio Bartorelli, MD, Fondazione Monzino, Milano, Italy, Luigi Badano, MD, U.O. Cardiologia, Udine, Italy, David Naftel, PhD., University of Alabama at Birmingham, Birmingham, AL, USA.

