Abstract
Aims: Percutaneous implantation of a large stent was performed in the coronary sinus of pigs, to assess safety and immediate efficacy for reduction of acute ischaemic mitral regurgitation.
Methods and results: Acute ischaemic mitral regurgitation (MR) was produced in seven pigs, continuously monitored with echocardiography, during repeated balloon inflations in the proximal left circumflex artery. The protocol was repeated following placement of a stent in the coronary sinus. Five pigs survived the period of acute ischaemia and developed severe mitral regurgitation (≥3+/4+). Following successful stent implantation, the MR area decreased from 2.4±0.4 cm2 to 1.1±0.6 cm2 (p=0.016) and the proximal isovelocity surface area (PISA) MR flow from 63.9±37.3 ml/sec to 44.0±35.0 ml/sec (p=0.029). Coronary sinus stent prevented the ischaemia-induced increase in septal-lateral mitral annulus dimension (p=0.041) and left ventricular dilatation. Three animals were allowed to recover and underwent histological analysis of the coronary sinus stent at 30 days, showing endothelialisation and minimal hyperplasia, without thrombus formation.
Conclusions: A percutaneously deployed stent in the coronary sinus may help to decrease the severity of acute ischaemic mitral regurgitation.
Introduction
Patients with heart failure often demonstrate several degrees of mitral valve regurgitation. The volume overload caused by mitral regurgitation may cause further deterioration of the haemodynamic strain of the failing left ventricle, leading to further negative remodelling, worsening of symptoms and adversely affecting survival1-3. One of the main causes of mitral insufficiency is enlargement, occasionally transient, of the mitral annulus, alone or in conjunction with structural valve abnormalities1. It has been hypothesised, that in patients with chronic ischaemic mitral regurgitation, the regurgitant orifice area can change dynamically in response to loading conditions, to changes in mitral annular (septal-lateral diameter) or left ventricular dimensions, and more specifically, to increased leaflet tethering and a reduced closing force of the mitral valve1. Until now, surgical annuloplasty has been the cornerstone of surgical mitral valve repair. Yet, this method, in selected patients, may be associated with considerable mortality and morbidity, including up to 28% recurrence of regurgitation4-8.
Over the last few years several percutaneous techniques are under development, using various principles, including the edge to edge technique9, coronary sinus annuloplasty devices10-14 with flexible or rigid elements causing partial pushing or traction of the mitral annulus, as well as other innovative designs employing radio-frequency induced annular constriction or suture-anchor plication through a magnet coupler.
Percutaneous methods with novel devices that take advantage of the proximity of the coronary sinus (CS) to the posterior leaflet and approximately 2/3 of the mitral valve annulus are intensely pursued. Via a catheter based percutaneous transvenous system, they aim to improve mitral leaflet coaptation, decrease septal-lateral mitral annular diameter and diminish mitral valve regurgitation11,12,15,16.
The purpose of our experimental study was to demonstrate the feasibility of percutaneous stent placement in the CS of pigs with acute ischaemic mitral regurgitation caused by temporary occlusion of the left circumflex artery (LCx) and the assessment of changes in left ventricular dimensions and volumes, septal-lateral annular distance and quantification of valvular insufficiency.
Methods
Animal preparation
Seven adult pigs, weighing 25-30 kg each, were studied according to the Principles of laboratory animal care as formulated by the National Society of Medical Research and the Guide for the care and use of laboratory animals published by the National Institutes of Health.
All animals were pretreated for one week with aspirin 325 mg and clopidogrel 75 mg daily. During the procedure animals were intubated and arterial blood pressure and electrocardiographic monitoring were continuously performed.
A 6 Fr introducer sheath was inserted in the right femoral artery and a 10 Fr sheath in the left femoral vein or the right internal jugular vein and intravenous unfractionated heparin (5000 U) was given. Coronary arteriography of the LCx was performed with a 6 Fr guiding catheter after intracoronary administration of 100-200 µg of nitroglycerin. Similarly, an angiogram of the CS was recorded after intubation of the CS with a 10 Fr guiding catheter. The diameter of the proximal LCx and the length and diameter of the mid segment of the CS were measured by online quantitative coronary angiography (QCA), using the coronary guiding catheter as a reference.
Acute ischaemic mitral regurgitation
Via the 6 Fr guiding catheter and a 0.014’’ angioplasty guidewire, ischaemic mitral regurgitation (IMR) was created by inflation of an appropriately sized angioplasty balloon (3.0 mm in diameter, 10 mm in length) at 10 atm in the proximal LCx for 5 min, during which the severity of the IMR was estimated by transthoracic echocardiography. In each animal the development of IMR and its echocardiographic evaluation was repeated three times, with 5 minutes in between intervals.
After CS stent implantation, the protocol was identically duplicated, with inflations of the same balloon at exactly the same coronary segment and IMR echocardiographic evaluation three times, with 5 minute intervals in each experiment.
Stent implantation
Following the initial sizing of the coronary sinus, an appropriately sized stent was selected. The distance between the CS ostium and the crossing point of the LCx was measured by QCA as the target zone, and the stent length was chosen (40 or 56 mm) to avoid LCx compression. The stent diameter we used was 8 mm or 9 mm, if the mid segment of the CS was less than 8 mm or 8-10 mm in diameter respectively. Under fluoroscopic guidance, a 0.038’’ guiding wire was introduced and a stainless steel 316 L stent (Zeus Endovascular stent, Medispes SW AG, Switzerland) was deployed at 10 atm. Optimal stent expansion was assessed angiographically. Intravascular ultrasound was not performed. Coronary angiography was repeated in order to exclude accidental occlusion of the LCx. A CS venogram was again performed to document the deformation of the CS, and to exclude any dissection or disruption after stent implantation, specifically at the edges of the stent.
Echocardiographic assessment
Transthoracic echocardiographic assessment, including two-dimensional and colour flow images, was obtained and analysed using a portable console (Vivid i, GE Medical System, USA and Europe) by an experienced echocardiographer. At baseline, the left ventricular (LV) dimensions (LV end diastolic and end systolic diameters and volumes), left atrial (LA) dimension, the largest diastolic septal-lateral mitral annulus diameter, the pre-existing MR, the maximal MR jet area, the transmitral flow and the LV outflow were measured. In addition, the degree of MR was estimated by MR jet area/LA area and proximal isovelocity surface area (PISA)17.
The same measurements were analysed and stored during each of the 3 cycles of balloon inflation and IMR before and after CS stent placement. Echocardiographic IMR parameters during the three inductions of coronary ischaemia, before and after CS stent placement, were averaged following off-line analysis.
Pathologic examination
In two animals the procedure was concluded after CS stent implantation, and the heart was immediately excised for gross pathologic examination. Three animals were allowed to recover and returned to the breeder. They continued aspirin 325 mg and clopidogrel 75 mg daily for 30 days, at which point they underwent euthanasia.
Gross histopathologic and microscopic examination was performed for evaluation of possible thrombus formation and endothelialisation of the CS stent as follows: the coronary sinus and stent were carefully cleaned of cardiac muscle; fixed in 10% buffer formalin for 18 hours; dehydrated in a graded series of alcohols; and embedded in methyl-methacrylate. Histological cross-sections 5-8 µm thick were obtained perpendicular to the long axis of the CS-stent using poly-cut heavy-duty microtome. Sections were stained with Goldner’s Trichrome stain. Histologic examination of the specimens was performed by light microscopy and two types of parameters were analysed:
1) Observational parameters, using a scoring system indicating the degree of histological changes (0=no changes, 1=mild, 2=moderate and 3=severe). The final score number corresponded to the mean of morphological changes at each strut site versus number of struts within that section. Such parameters are: neointimal inflammation; haemorrhage and presence of fibrin; peri-strut neoangiogenesis; injury of sinus wall; neointimal hyperplasia which represents neointimal response to injury and its appearance (homogeneously or heterogeneously encircling the lumen); and endothelialisation, which reflects the extent of the circumference of the sinus lumen covered by endothelial cells (1=25%, 2=25-75%, 3>75%).
2) Quantitative histomorphometric evaluation conducted by a commercially available semi-automated system. Parameters measured were: vessel area (mm2); stent area (mm2); adventitia (mm2); neointimal thickness measured in µm (the fraction of mean neointimal thickness at strut sites versus number of struts within that section); and endothelialisation index (the absolute number of endothelial cells divided by the lumen perimeter), reflecting the density of neointimal cells.
Statistical analysis
All values were expressed as mean±standard deviation. Paired t-test was used for statistical comparison of haemodynamic and geometric variables as appropriate. A value of p<0.05 was considered statistically significant.
Results
Seven pigs underwent the procedure of acute ischaemic mitral regurgitation. Two of them expired during the acute ischaemic phase from ventricular tachyarrythmias. In the remaining five animals, we did not observe any significant haemodynamic compromise or arrhythmia. The balloon occlusion, before and after CS stent placement, resulted in transient ST segment elevation and minor changes in heart rate and blood pressure. In these experiments a CS stent was successfully implanted, causing an angiographically obvious straightening of the CS (Figure 1).
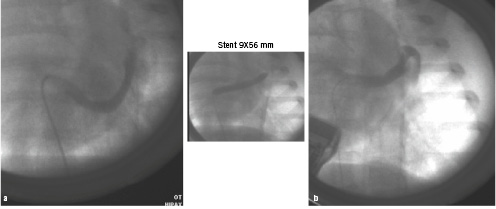
Figure 1. Coronary sinus venography before (a) and after (b) stent implantation.
The three animals that survived for 30 days at the breeder remained clinically stable without signs of congestive heart failure.
Echocardiography
LCx occlusion produced temporary development of 3-4+ IMR and posterior LV wall motion abnormalities before CS stent implantation in all experiments. At baseline, the left ventricular end-diastolic and end-systolic diameter and volume and the septal-lateral mitral annulus diameter were 3.68±0.3 mm, 2.68±0.40 mm, 34.0±0.7 ml, 14.2±3.1 ml and 2.93±0.62 mm respectively. Changes in these measurements during acute coronary ischaemia, before and after CS stent implantation, are presented in Table 1, demonstrating significant benefit of the CS stent in ischaemia-induced LV dilatation and annular enlargement.
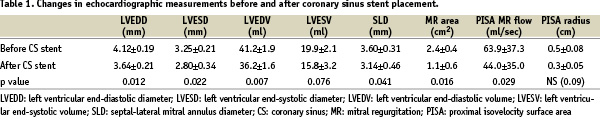
Following CS stent implantation, balloon occlusion in the LCx resulted in significantly less IMR, mainly in the regurgitation area and the PISA regurgitant flow (Table 1). We did not observe any LCx occlusion by the CS stent at the completion of the procedure.
Histopathological analysis
On macroscopic examination no evidence of thrombosis was identified in the coronary sinus. The stents were optimally expanded and endothelialised, without protrusion into the epicardial surface. In the surrounding tissues no signs of interstitial haemorrhage or oedema were detected. The ostia of the coronary arteries were patent and no evidence of thrombosis was noted. In the three animals allowed to recover, histology was performed at 30 days documenting endothelialisation of the coronary sinus stent without thrombosis (Figure 2).
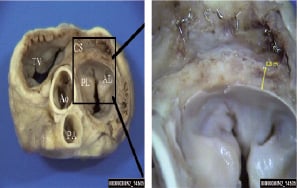
Figure 2. Partial and heterogeneous coronary sinus stent endothelialisation after 30 days.
The results of examined observational and quantitative pathologic parameters showed moderate and mainly heterogeneous stent endothelialisation. Briefly, the first animal showed minimal inflammation with no fibrin or haemorrhage, no indication of newly formed vessels (angiogenesis) or injury of elastic membrane. Re-endothelialisation was complete (Figure 3a) with evenly distributed neointimal hyperplasia (Figure 3b). The second and third animals showed moderate inflammation with little fibrin and haemorrhage, no neoangiogenesis or injury, however partial endothelialisation and heterogeneous neointimal hyperplasia were noted (Figure 3c).
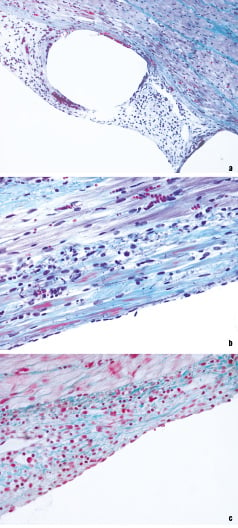
Figure 3. Histological images of the endothelialised stent struts using Goldner’s trichrome stain (X20) (a), with either complete (X40) (b), or partial (X40) (c) re-endothelialisation.
Quantitative parameters of the stented CS confirmed the differences between pigs regarding the homogeneity of neointimal growth, as well as the variability in neointimal thickness. The number of endothelial cells per lumen perimeter indicated dense distribution of cells in the first animal and relatively sparse in the second and third animal.
Discussion
Ischaemic mitral regurgitation often complicates myocardial ischaemia or infarction2. It is known that mitral regurgitation results in exacerbation of cardiac remodelling, leading to further dilatation and severity of MR, thus mitral insufficiency begets mitral insufficiency18,19. Our findings support the hypothesis that a CS stent may potentially limit LV dilatation caused by chronic ischaemic MR in patients with ischaemic cardiomyopathy. These results are in line with previous studies, reporting that surgical mitral annuloplasty eliminated MR in sheep with acute LCx ischaemia, mainly by reducing septal-lateral annulus diameter dilatation during ischaemia20,21.
The percutaneous annuloplasty approach utilises the anatomic proximity of the mitral annulus to the CS, located in the left atrioventricular groove, parallel, and occasionally in a small distance, to the posterior mitral annulus22. Previous experimental studies using different models demonstrated an acute or short-term improvement in either functional13,14,16,23, chronically ischaemic11,15 or acutely ischaemic MR12. Our findings coincide with these results and in particular with that of Liddicoat et al12 in a similar setting of acute ischaemic mitral regurgitation. This experimental model has been extensively used in preliminary studies to assess the effects of several annuloplasty devices. On clinical grounds, however, the selection of patients based on aetiology of MR, who might benefit from this procedure are not well understood. The development of MR is complex and multifactorial, caused by anatomic and/or functional abnormalities, such as LV enlargement, septal-annular dilatation, papillary muscle changes (acute ischaemia or rupture) and chordal deformations (shortening or abnormal elongation). Although speculative, the present technique may be more suited for patients, in whom chronic increase in septal-lateral diameter arises as the predominant mechanism for MR (chronic ischaemic or dilated cardiomyopathy).
The mechanisms responsible for improvement of cardiac performance following percutaneous annuloplasty are complex, including reduction of septal-lateral diameter and annular remodeling12,23, forward posterior leaflet movement with better coaptation11, as well as favourable changes in haemodynamic13,14 and neurohormonal parameters14.
Compared to stent placement, previously used anchoring devices in the CS may have the advantage of more distal positioning in the great cardiac vein11,12, thus potentially supporting a longer segment of the posterior annulus and, in some devices, a gradual shortening over 3-4 weeks to minimise vascular trauma11. Recent studies, however, in animals and humans, during this initial experience, reported notable complications such as coronary flow impairment14 and early device separation10. On the contrary, anticipated advantages of the CS stent concept include the potential durability of the device, the existing industry experience in intravascular scaffolding and the relative ease and familiarity of the interventional cardiologist in stent implantation. The intimal proximity of the CS to the LCx is highly variable, and any CS device implantation should be implemented with due care to angiographic documentation of appropriate anatomic landmarks, in order to avoid inadvertent coronary artery occlusion.
In this experimental study we demonstrated, that percutaneous stent implantation in the CS might serve as a device capable of limiting LV dilatation and reducing the IMR in pigs with acutely occluded LCx, mainly through preservation of the mitral septal-lateral annular diameter. This technique might alter the vicious circle of progressive IMR increase, as it reduces the circumference of the posterior mitral annulus and limits its motion towards the anterior mitral leaflet. Either mechanism may thus improve the mitral valve coaptation and reduce the MR.
Further studies are needed in order to develop a percutaneous safe and effective approach for the elimination of either acute or chronic IMR or functional MR24. Prevention of potential complications, such as LCx damage, device disruption and CS thrombosis should be specifically addressed by the novel device designs and their delivery techniques.
Limitations
Our findings should be interpreted in view of several significant limitations. The group of animals was small, however this was a preliminary observational study to assess the effect of coronary sinus stent on ischaemic MR. The findings were convergent, and as in previous small experimental reports, an identical protocol was carried out. The echocardiographic parameters were analysed by a single experienced echocardiographer. At 30-day follow-up, the surviving animals did not undergo repeat angiography, a limitation in our study, yet the coronary sinus stent was assessed histologically, and was optimally expanded. The use of transoesophageal or 3-dimensional echocardiography may have increased accuracy of MR detection and delineated the mechanism of the favourable effects more precisely.
Online data supplement
Echocardiographic moving images from an animal experiment, focusing on 2D/Doppler presentation of the mitral valve: (A) at baseline, (B) during acute coronary ischaemia with balloon occlusion of the left circumflex artery, causing ischaemic mitral regurgitation, and (C) during acute coronary ischaemia after placement of a coronary sinus stent, demonstrating significant decrease of inducible mitral insufficiency.

