Abstract
Aims: To describe the contemporary approach of chronic total occlusion (CTO) treatment of patients at the Thoraxcenter, Rotterdam, The Netherlands. Additionally, to make a critical appraisal of the performance of state-of-the-art CTO dedicated guidewires and devices in a prospective registry of patients.
Methods and results: During 20 months, a total of 160 consecutive patients (165 CTOs) were enrolled. The mean age was 61.5±11.1 years and 83.6% were male. In 91.5% of the patients this was the first attempt to open the CTO and 93.8% were de novo. The overall success rate was 60.6%. A median of 1 guiding catheter was used per case (Range: 1 to 9) and a median of 4 guidewires (Range: 1 to 11; 13 different types). 74.5% patients required more than one guidewire/device for the treatment of the CTO. The guidewires that most frequently crossed the CTO were the following: PT Graphix™ intermediate 33.0%, Miracle 3 g 27.4% and Crosswire NT 25.5%. The only device tested as a first option for the treatment of the CTOs was the CROSSER™. Overall, the CROSSER™ system was used in 23 (13.9%) patients with a success rate of 60.9%. The Point 9® X-80 Laser catheter was used in 10 (6.1%) patients with a success rate of 60%. Another 3 patients were treated with the Point 7® laser catheter. Both were used either to facilitate the crossing of the balloon, or to treat primarily in-stent restenosis occlusions. The SafeCross® System was used in 15 (9.1%) patients and the success rate in these patients was 46.7%. The most common strategy used in this registry was the use of an over-the-wire balloon in 81.5% of the cases. The parallel wire technique was used in 27.3% of the cases and in 12.7% was converted into a “see-saw” technique. When a large false lumen was created, re-entry into the true lumen was attempted in 21.2% of the cases, by means of IVUS guided approach and/or the use of stiffer guidewires, such as a Confianza guidewire. Retrograde recanalisation was attempted in 10 cases (6.1%), in three cases a graft was used; the remaining cases were treated either via collaterals or the septal branches.
Conclusions: The treatment of CTOs requires the use of a high number of guiding catheters and guidewires, as well as the use of sophisticated devices. The procedure must be carefully planned in advance as far as possible, as well as considering a prompt change in approach during the performance of the procedure to prevent complications derived from long procedures by using specific techniques such as parallel wire, see-saw, anchoring balloon, etc.
Introduction
Success in re-opening a chronic total occlusion (CTO) has significant benefits on quality of life as well as conferring a 10-year survival advantage compared with failed revascularisation1,2. There has been a significant increase in percutaneous procedural success rates for CTO over the last 10 years without an increase in adverse events2. Such successes have stimulated a shift from the conventional practice of treating all CTO patients with coronary artery bypass grafting (CABG) to a strategy that aims to treat these same patients with percutaneous coronary intervention (PCI).
Although a high proportion of CTOs may have a recanalised lumen that may facilitate the passage of a guidewire (GW)3,4, this passage of the wire across the missing section of vessel or ‘crossing’ of the lesion is still the most technically demanding phase of the procedure and is the predominant cause of failure. Therefore, many specific guidewires and devices have been introduced to try to overcome this most exacting phase of the CTO procedure. As a result, nowadays interventionalists are overwhelmed by the availability of multiple CTO-dedicated guidewires (stiffer, tapered-tipped and hydrophilic-coated guidewires) and also CTO-dedicated devices (Crosser™, FlowCardia, Inc., USA; Laser Guidewire®, Spectranetics, Colorado Springs, CO, USA; Safe Cross System®, Intraluminal Therapeutics, USA) commercially available for the treatment of the CTO. Some of them have been separately evaluated in randomised trials and registries5-7. However, most of the time the treatment of the CTOs requires a synergistic combination of components of this armamentarium. How do they perform? Do we have a predefined sequence of the use of these guidewires/ devices? To attempt to answer these questions, we prospectively collected data on 160 consecutive patients undergoing percutaneous treatment of a CTO at the Thoraxcenter, Rotterdam, The Netherlands. We describe in detail the tools and techniques used in the contemporary approach for the treatment of these patients.
Material and methods
Study patients
Those eligible for this study included all consecutive patients presenting with symptomatic coronary artery disease due to a chronic total occlusion. Chronic occlusion was defined as either an occlusion on angiography with no antegrade filling of the distal vessel other than via collaterals or minimal antegrade flow (TIMI flow 0 or 1)8,9. All patients included had a native vessel occlusion estimated to be at least one month’s duration based on either a history of sudden chest pain, a previous acute myocardial infarction in the same target vessel territory, or the time between the diagnosis made on coronary angiography and PCI. The type of CTO was either de novo or in-stent restenosis.
The protocol was approved by the local ethics committee and is in accordance with the principles of Good Clinical Practice for Trials of Medicinal Products in the European Community and the Declaration of Helsinki. All patients signed a written informed consent.
The key point of this registry was the presence of a dedicated research fellow present in the cath lab during all cases, registering every single manoeuvre, technique or approach applied in the treatment of the case. The two most experienced operators in the CTO procedures in our centre treated all patients.
Definitions
Biplane angiography
The use of two simultaneous fluoroscopic C-arms. The angiographic views should be orthogonal and should offer the best visualisation of the missing segment. Ideally, all cases with CTO should be treated in a cath lab with biplane assistance.
Bilateral injection
This improves distal visualisation of the target vessel by doing simultaneous injections in the right and left coronary system. The purpose is also to better localise the position of the tip of the guidewire in the occluded segment and to visualise the distal true lumen. To this aim, bilateral femoral access is established; the non-target vessel is usually engaged with a 5 Fr diagnostic catheter and if, during the procedure, the manipulation of the guiding catheter in the target vessel provoked frequent disengagement of the non-target vessel catheter, a guidewire was placed in the non-target vessel to maintain the catheter engagement.
Guided reopening of CTO: different modalities
Retrograde-guided PCI
When retrograde filling in the occluded vessel is sufficient, it is common to see the distal end of the occlusion. A guidewire can be distally placed through contralateral collaterals. This strategy has usually two purposes: first the GW can be left touching the distal cap of the occlusion as a landmark (“kissing guidewire”); secondly, an attempt to cross the occlusion retrogradely can be performed (Figure 1).
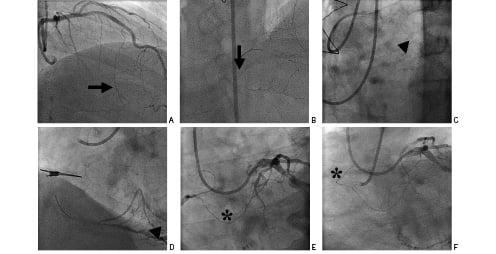
Figure 1. Retrograde technique. A and B via septal branch (arrow). C and D via saphenous vein graft (arrowhead). E and F via epicardial collateral (asterisk).
Intravascular ultrasound (IVUS)-guided PCI
This concerns the use of IVUS to facilitate the guidewire (GW), either to cross the occlusion or to re-enter into the true lumen. The former is possible when there is a big side branch at the entry point of the CTO, for instance when the CTO is in the ostium of the left anterior descending artery, the IVUS probe can be placed in the ostium of the left circumflex (and vice-versa) to guide crossing of the occlusion. The latter is possible when there is a large false lumen created after long GW manipulation without angiographic signs of perforation (Figure 2).
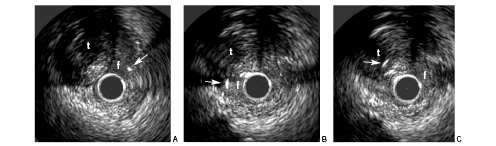
Figure 2. Panel A shows part of the vessel with the true lumen (t) at 11 o’clock. The arrowhead indicates the Crosswire NT guidewire in the false lumen (f) at 2 o’clock. Panel B shows the situation after manoeuvring the guidewire, still in the false lumen (f) now at 9 o’clock. Panel C shows the guidewire manoeuvred into the true lumen (t) at 10 o’clock. The IVUS probe is still positioned in the false lumen (f).
Multislice computed tomography (MSCT)-guided PCI
The 3-D volume-rendered reconstructed images produced by MSCT are imported into the Magnetic Navigation System (MNS) and overlaid on the fluoroscopic images (see also description of MNS below). Following this, a virtual lumen is identified using the vessel navigation tool that is a part of the MNS. This software marks points on both the MSCT and angiographic images using the “store marked position” option in the magnetic navigation software and these images are aligned using the alignment tool on the MNS screen. Finally, the vessel navigation software creates a virtual vessel overlay for the missing segment – namely the segment occupied by the CTO. After creation of the virtual vessel roadmap the operator can manoeuvre a guidewire through the vessel by indicating the desired point on a virtual roadmap that is synchronised to the fluoroscopic image (viewed from the same angle) and is displayed on a touch screen. The MNS computes a vector needed to navigate the guidewire and orientates the magnetic field that aligns the tip of the guidewire (Avi file 1).
Stent-guided PCI
This is is always the situation in cases of occlusive in-stent restenosis, where a permanent roadmap provided by the existing stent is present. This offers a particular situation where the interventionalist has instantaneous feedback of any manoeuvre performed with the guidewire.
Injection of contrast through the over-the-wire balloon (OTW)
Frequently, during CTO procedures there is uncertainty whether the GW is in the true lumen. The interventionalist can advance carefully the OTW balloon up to the tip of the GW, or as far as possible; then the GW is taken out and an injection of 1 or 2 cc of contrast media through the lumen of the OTW is performed. This allows you to visualise whether the GW is in the true lumen.
Improving support
In crossing CTOs it is essential to have optimal guiding catheter support; the following are some strategies to achieve this.
Guiding catheter selection
This is crucial in getting optimal support; for instance, in the treatment of the right coronary artery, an Amplatz left catheter was commonly used. Large size (7 or 8 Fr) guiding catheters are usually used in order to accommodate two balloons when there is the need for anchoring the balloon.
Stenting the proximal segment
When the proximal segment of the coronary artery is also diseased, stenting may facilitate deep engagement of the guiding catheter.
Anchoring technique
This consists of the placement of an inflated balloon in a non-target side branch10 (Figure 3).
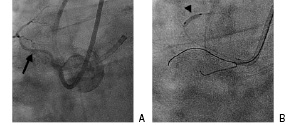
Figure 3. The anchor balloon technique. A. The arrow indicates the atrial branch of the Right Coronary Artery. B. A 2.0x12mm Maverick balloon (arrowhead) has been inflated in the atrial branch to improve guiding catheter support.
Child in Mother catheter technique or five-in-six system11
This is a method of inserting a 5 Fr guiding catheter into a 6 Fr or 7 Fr guiding catheter to increase backup support. Usually, the 5 Fr guiding catheter is 120 cm and the 6 Fr 100 cm long. The 5 Fr must have a soft tip to better negotiate any tortuosity it might encounter with the least possible damage (Figure 4).
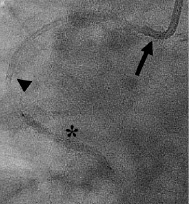
Figure 4. The “child-in-mother” technique. A 6 Fr mother guiding catheter (arrow) combined with a 5 Fr child catheter (arrowhead) facilitates delivery of a stent (asterisk).
Guidewiring of the non-target vessel
When one of the left coronary arteries is the target vessel a GW in the non-target vessel can be placed to increase support.
Manoeuvres to facilitate advancement of the balloon once the GW is placed distally
After crossing the chronic occlusion, the operator may find either that the balloon does not cross the occluded segment or that the occlusion is undilatable. Here are some strategies to facilitate the placement of the balloon in the occluded segment:
Crosser™
The Crosser™ CTO Recanalisation System (FlowCardia Inc, CA, USA) is mainly a CTO device for primary crossing of the lesion. However, it can also help when the lesion is undilatable. The Crosser™ system has been described elsewhere7,12. The catheter is monorail, hydrophilic, and 0.014 inch guidewire and 6 Fr guiding catheter compatible (Avi file 2). In this cohort of patients, in three cases the Crosser™ was specifically used to facilitate advancement of the balloon catheter.
Point 9® X-80 Laser catheter
There are two product configurations of the Point 9® X-80 laser catheter (Spectranetics, Colorado Springs, CO, USA): Vitesse (Rx) 110-004 or Extreme (OTW) 110-002. The Point 9® catheter has a 0.9 mm tip diameter and is 0.014 inch guidewire and 6 Fr guide catheter compatible. The maximum laser parameters are: 80 fluence, 80 hertz. In addition, a new Point 7® (0.7 mm tip) laser catheter has been also tested in this registry. The point 7 was used in 3 cases (Figure 5).
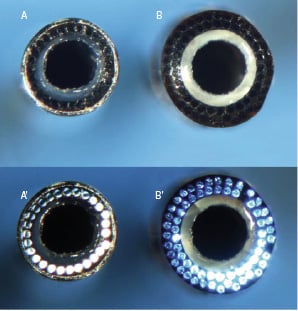
Figure 5. Point 7® (A) and Point 9® (B) laser catheters. In A’ and B’ the optical fibres are visualised. In the Point 7® laser catheter (A and A’) there is one single line of optical fibres, while in Point 9® laser catheter (B and B’) there are two lines.
Laser guidewire
The Laser guidewire® (Spectranetics, Colorado Springs, CO, USA) consists of a 0.018 inch shapeable guidewire containing 12 silica fibres with a 45 micron diameter. The guidewire was designed to function as an exchange guidewire. The fluence typically used during a laser guidewire procedure was 60 mJ/mm2, with a pulse repetition rate of 25 Hz. The laser guidewire was used in one case (Avi file 3).
“Open mouth” technique
This is used once the GW has broken the proximal cap of the CTO and it is not able to advance further. A balloon inflation in the proximal segment of the occluded segment results in a larger space, enabling CTO guidewires and CTO-dedicated devices to perform better. However, some complications may occur if the guidewire is in a false lumen such as perforation. Thus, it is recommended to perform in cases of totally occluded stents, where a permanent and visible roadmap is present and also when the operator is certain the guidewire is in the true lumen.
Over-the-wire balloon exchange
Commonly, CTO treatment includes, as a first choice, the use of an over-the-wire balloon to back up the guidewire. When the guidewire is placed distally, the OTW has to be pulled back to advance other balloons or the stent. To try to keep in place the guidewire while the OTW is being pulling back, an indeflator filled with saline is used. The indeflator is connected to the lumen of the OTW and then saline solution is irrigated at a constant pressure (roughly 16 atm), while the OTW balloon is being pulled back during fluoroscopy, to reduce the risks of GW displacement.
Parallel wire technique
This is the use of two guidewires for crossing the CTO, when one subintimal track has been created. With this method, the wire which enters the subintimal space is left there, and a second wire (stiffer than the first one) is inserted alongside it to find a new channel13 (Figure 6).
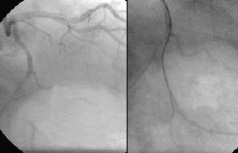
Figure 6. Parallel wire technique. In the right hand side panel the length of the occluded segment in the left circumflex was measured. Of note, the occlusion has a side branch at the entry point and ends in Y-bifurcation, these three angiographic characteristics have been associated with failure. On the left hand side, two guidewires are in place, both are clearly in a false lumen.
The see-saw technique
This also involves the use of two guidewires, but instead of leaving one of them in the false lumen, both are used sequentially to re-enter in the true lumen.
Partial reopening of the occlusion
Procedures where the GW crossed only half way through the occlusion and an important side branch was reached, but it was impossible to go further through the occlusion were considered a partial reopening.
Rentrop classification14
Grading of collateral filling was as follows: 0 = none, 1 = filling of side branches only, 2 = partial filling of the epicardial segment, 3 = complete filling of the epicardial segment.
Retrograde recanalisation
This is the opening of the CTO from the distal segment of the occluded vessel to the proximal segment of the occluded vessel. There are three possible routes to reach the distal cap: a) via arterial or vein grafts anastomosed to the native vessel distal to the site of occlusion in patients who have previously undergone CABG, b) via epicardial collaterals mainly between RCA and LCx through atrial branches or between distal RCA and LAD over the apex and c) septal collaterals. Bilateral arterial access is given for any route of retrograde approach (Figure 1).
SafeCross System15
The SafeCross System® (Intraluminal Therapeutics, Carlsbad, CA) is comprised of the Intraluminal guidewire, which is plugged directly into a console. The wire itself is 0.014 inch diameter and notably has a blunt tip; the distal 10 mm is radiopaque. The system uses optical coherence reflectometry to enable accurate guidance of the wire and a reduced risk of wire perforation. In addition, it enables the system to be forward looking with a very high resolution of up to 15 microns. The current Intraluminal guidewire has the capability of radiofrequency ablation with short-duration bursts (100 msec pulses) of low-frequency energy (250–500 kHz) delivered at the tip to enhance forward wire passage.
Venture® control catheter16
The Venture® is a 6 Fr, 0.014” compatible, 140 cm long over-the-wire, flexible and torqueable support catheter with a mechanically activated deflectable tip (90° degrees). It is intended to direct, steer, support, and control a guidewire (Avi file 4).
Magnetic navigation system
The use of a magnetic navigation system is a new option that may facilitate navigation in complex coronary anatomy17. The Stereotaxis Niobe® MNS has been extensively described elsewhere18. In brief, there are two permanent magnets positioned on either side of the fluoroscopic table. The MNS generates a composite magnetic field of 0.8 Tesla that is uniform in a 15 cm volume within the chest of the patient. This creates a magnetic field vector that can be rotated, translated and tilted in 360º in each plane, this allows fine control of the orientation of the tip of a magnetically enabled guidewire.
Statistical analysis
The analysis was done according to the principle “intention-to-use”, which means that all the manoeuvres and armamentarium employed during the procedure independently of the duration of the use were included. Continuous variables are presented as mean±SD and categorical variables are presented as counts and percentages.
Results
From October 2004 to April 2006, 160 (165 CTOs) consecutive patients were enrolled in this prospective, research-fellow witnessed registry of patients with a chronic total occlusion treated in a single centre. Five patients had two occluded vessels and were treated in the same index procedure.
The mean age 61.5±11.1 years, mostly being male 83.6% (Table 1).
For the majority of the patients (91.5%) this was the first attempt to open the CTO. Most of the occlusions were de novo lesions (93.8%), and the most frequently treated coronary artery was the right coronary artery (RCA) in 51.8% (Table 2). The angiographic characteristics are also shown in Table 2.
Guiding catheter selection
A total of 226 guiding catheters were used for the treatment of 165 CTOs. Overall, the median was 1 (Range: 1 to 9). Specifically, in the left anterior descending (LAD) and left circumflex (LCX) the median was 1 (Range: 1 to 9), whereas in the RCA the median 1 but the range went from 1 to 5.
For the LAD the first choice of guiding catheter was the Amplatz left 2 in 51.0% of the cases. While in the LCX the Judkins left guiding catheter was the most frequently used (41.1%). Lastly, in the RCA the Judkins right was the first option in 68.0% (Table 3).
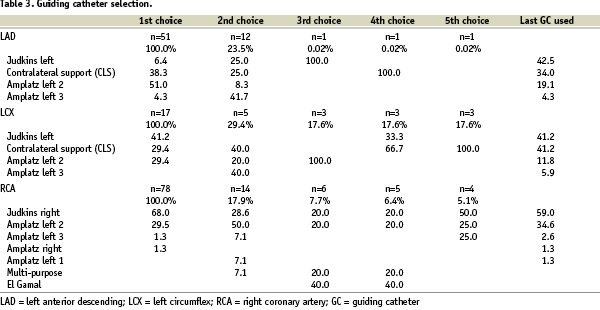
Of note, in the LAD, 23.5% of the patients required more than one guiding catheter; while in the LCX, 29.4% patients required more than one. Lastly, in the RCA, 17.9% patients required more than one guiding catheter.
Guidewire selection
Thirteen different types of guidewires were used. Overall, a total of 467 guidewires were used for the treatment of 165 CTOs. The median was 4 (Range: 1 to 11). Specifically, in the LAD the median was 3 (range: 1 to 11) and in the LCX the median was 1 (Range: 1 to 9), whereas in the RCA the median 2 (range: 1 to 11).
The first choice in 69.9% of the cases was the PT Graphix™ Intermediate (Boston Scientific Corporation, USA), followed by Miracle 3 g (ASAHI, INTEC, Co, Japan) in 12.9% of the cases and 5.5% of the cases were primarily treated using a Choice™ PT (Boston Scientific Corporation, USA). The magnetically enabled Cronus moderate support and Assert guidewires were used as the first option in 4 cases (Table 4).
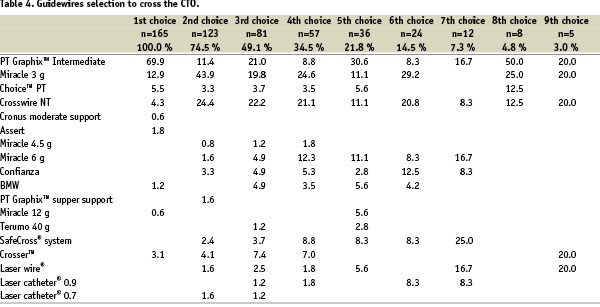
Seventy-four and a half percent (74.5%) of the patients required more than one guidewire/device for the treatment of CTO. Of note, as a second option, the PT Graphix™ Intermediate usage showed an important drop (11.4%), while the use of Miracle 3 g increased significantly (43.9%).
The guidewires most frequently used to cross the CTO in this registry were the following: PT Graphix™ Intermediate at 33.0%, Miracle 3 g at 27.4% and Crosswire NT at 25.5%. In total these three guidewires achieved 85.9% of the overall success rate.
Dedicated CTO device selection
Crosser™
Table 4 shows the frequency of the use of these different devices. The only device tested as a first option for the treatment of the CTOs was the Crosser™. Five patients were treated, in 2 patients with non-calcified CTO (multislice computed tomography analysis) the device was able to cross the occlusion from the beginning to end. The other three patients were successfully treated with a combined approach using Crosser™ and CTO dedicated guidewires. After the initial guidewire failed to cross the CTO, the most frequently used device was the Crosser™ (Table 4). Overall, the Crosser™ system was used in 23 (13.9%) patients with a success rate of 60.9%.
Laser
The Point 9® X-80 Laser catheter was used in 10 (6.1%) patients with a success rate of 60%. Another 3 patients were treated with the Point 7® laser catheter. Both were used either to facilitate the crossing of the balloon, or primarily to treat in-stent restenosis occlusions.
The Laser® guidewire was used in 7 (4.2%) patients as a last opportunity to cross the lesions; the success rate was 28.6%.
SafeCross® System
This system was used in 15 (9.1%) patients with a success rate of 46.7%.
Other devices
The Venture catheter was used in 4 patients (2.4%). Some issues were found with the use of this device. The steerability is far from being close to 1:1. There must be some room for the free movement of the device. On the other hand, it could be effectively used to direct the guidewire towards the entry point.
The magnetic navigation system was used in 5 cases (3.0%). Three cases were successfully treated by using multislice computed tomography coronary angiography guidance combined with highly precise guidewire steering (Magnetic Navigation System).
Rotational atherectomy was unsuccessfully used in 1 case (0.6%) to allow the passage of the balloon.
Techniques to open CTOs
Many techniques and strategies were employed in the treatment of these patients. The most common in this study was the use of an OTW balloon in 81.5% of the cases. Bilateral injection was used in 63.5% of the procedures. The parallel wire technique was used in 27.3% of the cases and in 12.7% was converted into a see-saw technique. When a large false lumen was created, re-entry into the true lumen was attempted in 21.2% of the cases, by means of IVUS-guided approach and/or the use of stiffer guidewires, such as a Confianza guidewire. The open-mouth technique was used in 9.8% of the cases. A contrast injection through the OTW was performed in 4.9% of the cases.
Retrograde recanalisation was attempted in 10 cases (6.1%), in three cases a graft was used; the remaining cases were treated either via collaterals or the septal branches.
Forty-six patients (26.1%) in this cohort underwent MSCT scanning before the CTO procedure.
The mean volume of contrast used was 457.5±191.6 ml. The mean procedure time was 128.0±58.9 min, with a fluoroscopic time of 67.2±44.7 min and radiation exposure of 12,395.9±8,320.7 DAP.
It is worth mentioning that the mean overall cost of the procedure was 5,544.90±3,366.20 Euros, including stent cost.
Discussion
The invasive treatment of CTO by means of PCI is still one of the most challenging procedures for the interventional cardiologist. It is also one of the most unpredictable procedures as well. The original intended strategy is usually changed during the procedure according to the different issues the operator may encounter. Thus, there is no universal approach capable of successfully treating this subset of lesions. However, there are some guidelines that can help us to easily and smoothly overcome these issues. This paper reports, in detail, a single-centre’s experience in the treatment of chronic total occlusions using nearly all the current available guidewires, devices and techniques. This report attempts to be a narrative of these real time CTO procedures. There is growing evidence in favour of opening a CTO1,2,6, but this benefit must be balanced by the potential disadvantages for the patients which may arise from all these multiple issues that come together with the procedure itself. Even when there is no comparative group, it appears that treating CTOs using these types of procedures are more challenging, time consuming, risky (in terms of radiation safety, contrast use and the risk inherent in the employment of specific CTO dedicated techniques/guidewires/devices) and expensive than any of the others in the field of interventional cardiology. For instance, if only the contrast medium is considered, in this cohort alone there was, on average, 457.5 ml used. It has been reported that for each 100 ml there is an increment in the overall risk of contrast-induced nephropathy (CIN); therefore in this population, on average, the risk of CIN would be thus 7.5%, with the risk of dialysis of 0.04%19. Comparing the mean contrast used in this study (457.5 ml) with recently published data on non-chronic occlusions – as reported by Marenzi et al20 where the mean contrast used was 274±113 ml, or in the report by Le Feuvre et al21 where the mean contrast used were 267 ml and 276 ml in the two studied groups – it is clear that in considering this all comers registry of those treated for chronic total occlusions that the contrast use is much higher.
In addition, long procedure times and high radiation exposure still are the sine qua non in the PCI of the CTO patients. Reduction in total radiation dose might prevent acute and long-term radiation effects such as skin injuries22.
From the hardware development point of view, CTO dedicated guidewires/devices have gone through several waves of technological advances in the past few years in order to meet the very high technical demand required by this type of lesion. Saito et al23 reported on the comparison between two clearly defined periods, which differed mainly due to the use of tapered tip guidewires in the latest period; with a change in success rate that was documented from 67% to 81%. Thus, the introduction of these guidewires has been one of the major breakthroughs in the treatment of CTO lesions. With respect to this report, it is clear that our centre is in a period of transition from the use of standard/stiffer guidewires to the early use of tapered tip guidewires. As far as the approach is concerned, one of the challenges lying ahead is the retrograde approach, which offers a kind of dual alternative in the same patient. In other words, when the antegrade approach has been unsuccessful, the retrograde is still an option under certain conditions in carefully selected patients. This approach is technically demanding and requires not only operator skills but also special equipment. In parallel, we have explored some other novel alternatives as the combined approach of the use of MSCT and magnetic navigation, integrating the actual images of the coronary tree (MSCT images) of the patient into the MNS software for use as a roadmap, and the ablation capabilities of some of the CTO devices such as the SafeCross® system and laser. These three components constitute for us the ideal trifecta (forward looking, highly precise steerability and ablation capabilities) needed to treat CTOs. In non-calcified and non-tortuous CTOs we have also assessed the performance of the Crosser™ system in a few cases with encouraging results that prompted us to start a registry of CTO patients treated with this system as a first option.
In these long-lasting procedures it is important to consider any short-cut to save time, effort and more importantly to reduce risks, for both the patient and the operator. Therefore many techniques described in the methods section could help us reach this goal. One important lesson is the early use of tapered tip GW during the procedure: the operator should not waste time using stiffer hydrophilic guidewires if a large false lumen has been created or non-significant progress has been made, instead a tapered tip GW should be used.
The success rate is still far from being optimal. However, now that we have a deep insight into our way of treating these patients, several actions have been started to launch a new approach to treat these patients, mainly with the widely use of tapered tip GWs and the retrograde approach.
Conclusion
The treatment of CTOs is more complex that we had thought. It requires the use of a high number of guiding catheters and guidewires, as well as the use of sophisticated devices. Pre-procedural evaluation of the CTO lesion by means of multislice computed tomography is becoming routine. Above all, the procedure must be carefully planned in advance – as far as possible – taking into consideration, as well, the possible necessity of promptly changing this approach during the procedure in order to prevent complications derived from its lengthy duration, and accomplishing this by using specific techniques such as parallel wire, see-saw, anchoring balloon, etc.
Online data supplements
Video 1. Imaging co registration (MSCT on angiography for the treatment of CTO)
Video 2. The Crosser™ device
Video 3. The laser wire
Video 4. Venture steering catheter

