Abstract
Aims: To verify feasibility and safety of bone marrow stem cells (BMC) mobilization in patients (pts) with acute myocardial infarction (AMI) and to monitor the clinical effects of BMC mobilization in terms of myocardial perfusion and function.
Methods and results: Eight male pts (median age: 50.5 years) treated with a primary PTCA were enrolled. The mobilization regimen consisted of G-CSF 5 µg/kg/12 h from day 0 to day +2 and GM-CSF 2.5 µg/kg/24 h. All pts underwent coronary angiography, intracoronary doppler flow study, echocardiography, and nuclear thallium scan before treatment and at 6 months. All pts showed increased values of WBC and circulating CD34+ following cytokine administration. No patient died. All patients completed a 6-months follow-up: target lesion revascularization rate was 12,5%, target vessel revascularization rate was 37.5%, angiographic mean ejection fraction increased from 49.8±11.9 to 55.4±8.7 (p=NS), mean coronary flow reserve from 1.63±0.42 to 2.5±0.4 (p=0.001), mean Thallium uptake raised from 55.56±16.42% to 67.56±13.66% (p=0.01), and normally perfused segments from 16% to 52% (p=0.01).
Conclusion: Cytokine-induced BMC mobilization is feasible in AMI pts. Improvements of myocardial perfusion can be expected after PTCA associated with G-CSF and GM-CSF induced mobilization. Further studies are required to define the role of BMC-mobilization and the most effective cytokine combination.
Introduction
Recent preclinical and some clinical studies have showed feasibility and a potential clinical benefit of cell therapy in acute and chronic coronary syndromes1-8. Results obtained both in animal models and in humans suggest that myocardial regeneration and neovascularization of ischemic myocardium may occur following intracoronary or intramyocardial injection of bone marrow-derived cells (BMC)9-14. Cell therapy is viewed with increasing interest in the management of coronary artery disease, in particular acute myocardial infarction (AMI).
So far, the most widely employed procedure includes the aspiration under general or local anesthesia of autologous BMC, their isolation and their re-injection into the affected heart. An alternative approach may be the mobilization of BMC into peripheral blood (PB). Cytokine-induced mobilization is an appealing approach, because it is easy, non-invasive and potentially effective. The prolonged PB circulation of high levels of stem and progenitor cells might favour BMC colonization of AMI-related segments. Furthermore, the observation of variable amounts of circulating BMC in patients with AMI suggests that the mobilization phenomenon is a naturally occurring process in response to physiopathologic conditions like ischemia and infarction15-18.
Several cytokines including GM-CSF and G-CSF, routinely used in haematological malignancies for BMC transplantation purposes, are known to have potent mobilization capacity. Some preclinical studies have shown potential benefits for G-CSF administration in ischemic conditions, in terms of myocardial repair and microvascular enhancement19-20. On the other hand, few clinical studies have been performed, with some controversial results in terms of safety and efficacy21-25. We here report preliminary results of a pilot study on BMC mobilization induced by the concurrent administration of G-CSF and GM-CSF in patients with AMI treated with primary PTCA. The purpose of this phase 1 study was to verify feasibility and safety of this novel treatment approach.
Methods
Study population
The study group comprised 8 patients with AMI treated by primary PTCA. The inclusion criteria were typical chest pain lasting > 30 minutes and ST-segment elevation of ≥ 1 mm in ≥ 2 contiguous lead, admission within 12 hours from the onset of symptoms and a coronary artery lesion suitable for primary PTCA with stenting. Exclusion criteria were age ≥ 75 years, cardiogenic shock, previous myocardial infarction, hematopoietic disorders or splenomegaly, hepatic or renal failure, malignancy, pregnancy or infectious diseases. All eligible patients were enrolled in the study if they gave formal written informed consent. The study was carried out according to the Declaration of Helsinki. The protocol was approved by the Regional Ethical Committee.
Study protocol
All patients underwent primary PTCA according to current international guidelines26. Before PTCA, patients were treated with aspirin, i.v. nitrates and a 5000 IU bolus of unfractionated heparin. Coronary angiography was performed using a femoral approach. Coronary stenting was performed using standard procedures and the IIb/IIIa inhibitor abiciximab was used during procedure if needed. Betablockers, angiotensin-converting enzyme inhibitors and statins, if not contraindicated, were routinely administered. At 24 hours after completion of intervention, patients were started on G-CSF (Myelostim, kindly provided by Italfarmaco) at 5 µgr/kg s.c. twice a day for 3 consecutive days (from day 0 to day +2) and GM-CSF (Mielogen, kindly provided by Schering-Plough) at 2.5 µgr/kg s.c. once a day for 5 consecutive days (from day 0 to day +4). Since transient severe hypotensions was noticed in the first three patients, the protocol was amended by reducing and tapering GM-CSF administration to 2.5 µg/kg once a day at day +2, +4, +6. Postinterventional antithrombotic therapy consisted of ticlopidine 250 mg bid for 4 weeks or 6 months, respectively if bare metal or drug eluting stents were used. Enoxaparin was administered (4,000 IU or 6,000 IU if patient weight was respectively < 80 Kg, ≥ 80 Kg) during BMC mobilization, as long as the WBC count was ≥ 20,000/mm3.
Clinical and metabolic data
All patients were screened for symptoms, signs and ECG changes baseline, during hospital stay and at 30-days and 6-months follow-up. Cardiac enzymes, C-reactive protein, hemocromocytometric and coagulation parameters, hepatic and renal function parameters were monitored daily during the hospital stay. All adverse clinical events were recorded during the hospital stay and at the 6-months follow-up. Major adverse clinical events (MACE) were defined as death, re-infarction, ventricular arrhythmias, recurrent angina, heart failure, target lesion revascularization (TLR) and target vessel revascularization (TVR).
Quantitative coronary angiography and coronary blood flow
All patients underwent quantitative coronary angiography, left ventricular (LV) angiography, doppler coronary blood flow baseline studies (immediately after PTCA) at a 6th month interval. Images of coronary angiograms and LV angiograms, acquired by a single-plane left ventriculography in the 30° right anterior oblique view, were analysed with the Integris H5000C system. Volumes and global LV ejection fraction were determined by the area-length method. All lesions in any branch of the left and right coronary system were investigated with an intracoronary Doppler flow study, immediately after completion of the PTCA intervention, if the distal vessel was without significant disease. Coronary flow velocities in the stented segment were mesured with a 0.014 Doppler FloWire and analysed by the FloMap system (Cardiometrics Inc.). The tip of the Doppler wire was positioned at the proximal end of the stented segment to ensure a reproducible sampling site without any vasomotor changes. Doppler flow velocity spectra were analysed in real time to determine time-averaged peak velocity. Basal and peak coronary flow velocities were determined 5 to 10 seconds after intracoronary bolus doses of adenosine (18 µg for the left coronary artery and 12 µg for the right coronary artery). Coronary flow reserve (CFR) was defined as the ratio of maximal flow to corresponding baseline flow. At 6 months after the initial intervention, coronary and LV angiography and flow velocity measurements were repeated. If indicated, a further PTCA was performed before the second flow velocity measurement.
Single photon emission computed tomography follow-up
All patients underwent a myocardial perfusion SPECT study according to a rest-redistribution protocol 24 hours after primary PTCA, repeated 6 months later. Immediately, and then 4 hours after the i.v. administration of 111 MBq of 201 Tl, images were acquired using a dual detector gamma camera. Horizontal and vertical long axis views were obtained. Short axis slices from the first apical to the last basal one were used for generating a two-dimensional 16 segment polar map, close to the echocardiographic analysis. A semi-quantitative analysis was then performed: the segment with the highest mean tracer uptake was normalized to 100% and the activity within the other segments was expressed as a percentage of peak activity. Segmental 201 Tl activity was classified as severely abnormal if it was <50% of the peak value, mild to moderately abnormal if it was >50% and <65% of the peak value27.
Echocardiographic follow-up
An echocardiographic study was conducted 24 hours after primary PTCA and 6 months later. Echocardiographic images were obtained using a scanner (HP Sonos 5500), equipped with a 2.5-3.75 MHz transducer. Images were recorded to identify the asynergic area and to quantify both left ventricular ejection fraction (LVEF) and wall motion score index (WMSI). The left ventricle was divided up using a 16 segment model; the wall motion of each segment was scored according to a 5-point scale. A WMSI was obtained averaging the scores of the visualized segments. Left ventricular ejection fraction was calculated by using the modified Simpson’s rule. The analysis of 2D echocardiograms was performed by two independent observers.
Haematologic procedures
All patients were evaluated to rule out any underlying haematological disease. Their clinical presentation and cell blood count (CBC) were monitored daily. According to protocol rules, cytokine administration had to be discontinued with WBC values higher than 50,000 per µL or in the presence of severe adverse clinical manifestations. BMC mobilization was monitored by daily evaluation of circulating hemopoietic stem and progenitor cells assessed as CD34+ cells as well as in vitro clonogenic cells (CFU-GM assay), during cytokine administration and for a few days thereafter. The CD34 assay was performed on whole blood by cytofluorimetric analysis using the monoclonal antibody anti-CD34 HPCA-2 (Becton & Dickinson, San Jose, CA), as previously detailed28. Briefly, 100 µL of heparinized whole blood was incubated with 10 µL of anti-CD34 PE for 30 minutes at room temperature in the dark. Red cells were removed by hypotonic lysis with NH4Cl buffer for 15 minutes in the dark, followed by two washes with PBS supplemented with 1% BSA and 0.1% sodium azide. Cells were then resuspended in PBS and analysed with a FACSCalibur cytofluorimeter (Becton&Dickinson), equipped with a filter set for FITC-PE dual-color fluorescence. Data acquisition and analysis were performed with the CELLQuest Software and each measurement included at least 40,000 events. The number of circulating CD34+ cells was obtained by multiplying this percentage by the total number of leukocytes in 1 µL of blood. Circulating myeloid (CFU-GM) progenitors were evaluated daily as well28. The CFU-GM assay was carried out as follow: 0.5 to 2 x 105 nucleated cells were plated in 35-mm Petri dishes in methylcellulose-based medium (HCC-4100; Stem Cell Technologies, Vancouver, Canada) supplemented with rhSCF, rhIL-3, rhG-CSF and rhGM-CSF (Stem Cell Technologies). Progenitor cell growth was evaluated after 14-18 days of incubation. A median value of 146 CFU-GM/ml was obtained in unstimulated PB from normal donors.
Study end points and measures of clinical outcomes
The primary end point was to verify feasibility and safety of BMSC mobilization. We monitored all major adverse events (death, re-infarction, major ventricular arrhythmias, recurrent angina, heart failure, target lesion revascularization, target vessel revascularization) from the admission to the following 6 months. As secondary end points, we analysed the clinical effects of cytokines induced stem cells mobilization in terms of global and segmental myocardial perfusion and function.
Statistical analysis
Categorical data are presented as absolute values and percentages, whereas continuous data are summarized as median or mean value ±SD. The Chi-squared test was used for comparison of categorical variables. Comparison of continuous variables was performed by means of t-test for paired or unpaired samples, as appropriate. A value of p<0.05 was regarded as significant.
Results
Main patient characteristics and early clinical outcome
Between September and December, 2003, 8 consecutive patients have been enrolled in the study program. Their main clinical, angiographic and procedural characteristics at presentation are reported in Table 1.
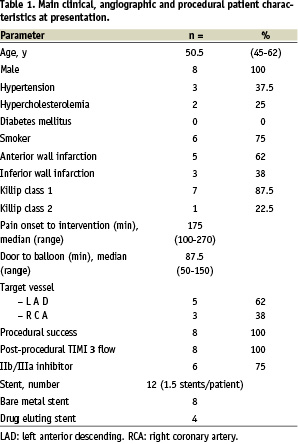
The first 3 patients enrolled, showing large anterior AMI, experienced persistent hypotension requiring IABP, in one patient associated with atrial fibrillation and in another patient recurrent angina. On the basis of this observation, the protocol was amended by reducing and tapering GM-CSF administration, as mentioned above. Hospital course was uneventful in following the 5 patients. No death occurred during in-hospital stay and all patients were discharged between between 9 and 14 days (median 10.5) after admission. All patients showed increased values of WBC and circulating CD34+ following cytokine administration, with median peak values of 33,925 WBC/µL (range: 21,100 - 47,700) and 28 CD34+/µL (range 9.3-70), recorded at days +3 and +4 respectively, as shown in Figure 1.
Figure 1. Changes in WBCs and circulating CD34+ve cells and CFU-GM following concurrent administration of G-CSF and GM-CSF.
Each point indicates median values with range. All patients received G-CSF (Myelostim) at 5 µgr/kg s.c. twice a day for 3 consecutive days (from day 0 to day +2); GM-CSF (Mielogen) was given concurrently at 2.5 µgr/kg s.c. once a day, from day 0 to day +4 in the first 3 patients and then once a day on day +2, +4, +6 in the subsequent 5 patients.
Both WBC and circulating CD34 rapidly decreased towards normal values within 3 days after cytokine discontinuation. Circulating CFU-GM were increased as well, with median peak value of 4,260 µL (range: 1,800 - 10,400) recorded at day +3 (Figure 1).
Late clinical outcome
All patients were alive at 6 month. The angiographic follow-up showed a TLR and a TVR rate respectively of 12.5% (1 patient) and 37.5% (3 patients). No other MACE were detected during the same time course.
Regional and global LV function
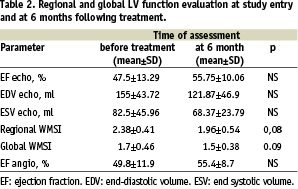
The echocardiographic and LV angiographic analysis were completed in all patients and showed a 6 month statistically non-significant improvement of mean ejection fraction and mean systolic and diastolic volumes. A 6 month non-significant improvement of regional and global WMSI was also detected (Table 2).
Myocardial perfusion invasive and non-invasive data
Myocardial perfusion results are reported in Table 3.
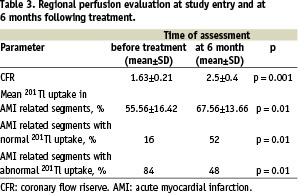
Basal and peak coronary doppler flow velocity analysis was completed in all patients and showed a 6 month statistically significant improvement of CFR (1.63±0.21 versus 2.5±0.4, p=0.001). Nuclear Thallium scan study was completed in 7 patients (87.5%). A total of 25 AMI related segments were evaluated. As detailed in Table 3, the SPECT analysis showed a significant recovery of myocardial perfusion at 6-months follow-up, along with a significant increase in the proportion of normally perfused segments.
Discussion
Despites substantial advances achieved in the reperfusion treatment of AMI patients, including primary PTCA and pharmacological adjunctive therapy, left ventricular dysfunction is still a frequent complication after AMI. The variably impaired ventricular function may have a significant negative impact on long term survival. Several studies have been performed to develop novel treatment strategies for preventing LV remodelling and ultimately improving the long term outcome of AMI patients. In particular, cell therapy has been considered as a possible tool to reduce LV remodelling and preliminary results have been promising1-14. So far, myocardial stem cell therapy has been most frequently attempted by either epicardial or transcatheter endocardial or intracoronary cell injection. Both the epicardial and endocardial approaches can not be accomplished in the acute phase of myocardial infarction. Also the intracoronary route has several restrictions, including the cost and complexity of the procedure that preclude a widespread use of this approach.
In trying to simplify the cell therapy-procedure for AMI patients, an innovative approach based on cytokine-induced BMC mobilization was designed. Cytokines BMC mobilization might be an effective option and it is simple and non-invasive. Indeed, some preliminary studies have been already performed with G-CSF given for mobilization purposes during AMI. However, differing results have been reported, about either efficacy or safety, and it is still uncertain which cytokine or combination of cytokines could be the most effective and safest21-25.
G-CSF is widely employed in the management of haematological diseases. Its potent mobilizing activity and high tolerability are well known28-29. Indeed, G-CSF is the usual drug employed in normal volunteer donors in order to induce peripheral blood stem cells mobilization for marrow cell transplantation30. Due to these features, G-CSF has been also employed in some preliminary experiences aimed to exploit the mobilization process for the regeneration of non-haematological tissues. In our study, G-CSF was administered in combination with GM-CSF. This latter was included because of its reported mobilizing activity on endothelial precursors31. Indeed, a strong arteriogenic activity mediated through amplification of monocyte function has been attributed to GM-CSF32. Furthermore, previous observations have shown that large amounts of very immature haematopoietic progenitors are mobilized when GM-CSF is given after chemotherapy33. These features prompted us to design an original mobilization protocol combining low-dose GM-CSF with standard dose G-CSF.
Our phase 1 study is the first designed using both G-CSF and GM-CSF in the acute phase of myocardial infarction. The analysis of feasibility and safety of G-CSF and GM-CSF BMC mobilization in AMI was our primary end point.
Severe, though transient, hypotension was observed in the first three patients. This haemodynamic phenomenon could be secondary to a vasodilating effect of GM-CSF and related to an excess of bradikinin-callicrein system activation. Since then, GM-CSF doses were reduced and no other severe side effects occurred in the remaining 5 patients. Indeed, the combined administration of G-CSF and GM-CSF at reduced dose proved to be feasible in the acute phase of myocardial infarction, without severe adverse reactions and all patients were discharged alive.
A marked leukocytosis was regularly recorded following cytokine administration, with maximal peak values below 50,000 WBC/µL in all patients. Furthermore, a variable degree of BMC mobilization was always seen, although peak values of circulating CD34+ cells were quite variable among the 8 patients and overall lower than those commonly recorded in normal volunteer donors30. The demanding cardiac condition and/or some of the various drugs administered in the acute phase of myocardial infarction might be the reasonable explanation of the reduced mobilization capacity observed in our series of AMI patients.
The main issue of concern while administering cytokines in AMI patients is the risk of coronary stent restenosis. A recent trial had to be discontinued following the occurrence of in-stent restenosis in the first 3 patients enrolled in the study24. In our study, a binary restenosis rate of 12.5% was observed at the angiographic control performed at 6 months. This is in line with the restenosis rate routinely seen at our institution as well as with the rates reported from large series of primary PTCA programs34. Thus, our data does not confirm the very high rate of restenosis of the MAGIC trial24. This discrepancy could be explained by the different treatment schedule. In our study, primary PTCA was immediately performed on patient admission and cytokine administration was started 24 hour later. On the contrary, PTCA was delayed, until after maximal G-CSF stimulation in the MAGIC study. It seems likely that the risk of in-stent restenosis might be markedly increased if intracoronary stenting is performed at maximal G-CSF induced-leukocytosis.
In spite of the low restenosis rate, atheromatous disease progression in coronary segments distantly from the site of stenting occurred in 3 patients (37.5%). This is a somewhat high incidence of atheromatous disease progression compared to what is commonly observed in patients undergoing PTCA for coronary artery disease34. This observation was unexpected and could be a chance finding. However, cytokines might have contributed to the progression of silent coronary lesions. The well known pro-inflammatory activity of GM-CSF, exerted through monocyte-macrophage stimulation, may have favoured a further activation of the so called clinically silent vulnerable plaque35. Evaluation of a larger series of patients is needed to define the actual incidence of atheromatous disease progression in AMI patients undergoing BMC mobilization and the role of cytokines in promoting the activation of vulnerable plaques.
All patients were alive and well at 6 months since AMI and they could be evaluated for their long-term heart function. A significant increase of both coronary flow reserve and thallium segmental uptake was observed, along with some improvements, though not statistically significant, of left ventricular volumes and ejection fraction. At present it is hard to ascertain whether improvements of myocardial perfusion should be ascribed merely to the PTCA or to cytokines administration and to BMC mobilization or to the combination of all these factors. One might assume that the combined administration of G-CSF and GM-CSF might have boosted the circulation of early precursors, that have been found at increased levels in the early phase following AMI15-18. These precursors might have favoured the neovascularization and possibly the regeneration of myocardial tissue. Further randomised studies are now in progress to verify the true benefit of cytokine administration and BMC mobilization early after PTCA in AMI patients.
Aknowledgements
We are deeply indebted to Dr. Ronald Vlietstra for revising the manuscript and his precious suggestions.
This work was in part supported by grants from Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) and from Regione Piemonte - Ricerca Finalizzata.
G-CSF (Myelostim) was kindly provided by Italfarmaco, GM-CSF (Mielogen) was kindly provided by Schering-Plough.
Excerpt from the reviewers
Although this manuscript does not provide much in the way of “earth-shattering” new data, it does address an important clinical question; the safety of the administration of bone marrow mobilising cytokines in acute coronary syndromes. The trial design is a phase I clinical trial enrolling only 8 subjects. Because of the small sample size and trial design, we cannot expect much in the way of meaningful data. However, the authors do prove their primary endpoint; the feasibility of the administration of these cytokines in the setting of an acute myocardial infarction. They also show that, although safe, the administration of G-CSF and GM-CSF may promote atherogenesis. Although several abstracts presented at major international meetings have suggested that cytokine administration may in fact promote atherogenesis in preclinical models, there is only one paper published in a peer reviewed journal which suggests an anti-atherogenic effect of GM-CSF.
It is difficult to criticise a well designed trial such as this. The authors have addressed their primary endpoint, and have discussed their findings well. I feel that the findings of Marra et al do make a significant, albeit small, contribution to the field of cell based therapy for the treatment of ischemic cardiac conditions.

