Abstract
Aims: Percutaneous aortic valve replacement is a novel technology with great promise for the treatment of aortic valve disease. However, concern has been raised regarding complications associated with this cutting-edge procedure.
Results: We reviewed the experience in Europe, Canada, and the United States with the Cribier-Edwards™ Bioprosthesis in order to identify complications associated with percutaneous aortic valve implantation. Complications may be associated with the implantation technique or the device itself. The antegrade approach may be associated with mitral valve leaflet tethering and trauma while the retrograde approach is primarily associated with vascular complications. Complications associated with both techniques include device embolization, paravalvular aortic regurgitation, and possibly coronary ostia obstruction. These complications, as well as strategies to minimize them, are discussed.
Conclusions: Percutaneous aortic valve replacement is an innovative yet provocative technology. Although dramatic successes have been realized, several novel complications and limitations have emerged. It is encouraging that these problems are being addressed with refinements in technique and device development which ultimately will enhance the safety of this procedure.
Introduction
Percutaneous aortic valve replacement is an exciting technology, still in its infancy, with the potential to revolutionize the care of patients with aortic valve disease. The first percutaneous aortic valve implant was performed by Cribier in 2002.1 Since then investigators in Europe, Canada, and the United States have implanted over 80 valves using the Cribier-Edwards Bioprosthesis™ (Edwards Lifesciences, Irvine, CA).2 A second percutaneous heart valve (PHV), CoreValve’s ReValving™ System (CoreValve, Paris, France and Irvine, CA), has been implanted in the aortic position in humans and is gaining more experience. Numerous companies and investigators are exploring other novel technological options. The initial experience has been promising, at times with spectacular clinical results, but not free of complications. In fact this new technology is associated with procedural and device related issues that must be surmounted as the field is advanced forward. This paper describes complications associated with the Cribier-Edwards Bioprosthesis™, the first percutaneous aortic heart valve (PHV) placed in humans and the device with the largest worldwide experience. The Cribier-Edwards Bioprosthesis™ is a trileaflet bovine pericardial valve mounted within a stainless steel, tubular slotted stent which is 14.5 mm in height and 23 mm or 26 mm in external diameter. There are two methods for PHV implantation: the retrograde and antegrade technique. Complications unique to each approach, as well as those common to both, are discussed.
Balloon aortic valvuloplasty
Balloon aortic valvuloplasty (BAV) is performed prior to PHV implantation in order to create a channel through which to pass the PHV. Therefore, the complications of PHV implantation necessarily occurs against the background of BAV complications, which are not inconsiderable. The initial studies of BAV showed mortality rate of 3.0% to 7.5%, stroke 0.4 to 4.6%, myocardial infarction 0.2 to 1.0%, and cardiac perforation 0.3 to 1.8%.3-8 A recent retrospective analysis of 103 critically ill patient, one-third of whom were in shock, treated with BAV at William Beaumont Hospital revealed a mortality rate of 15%, stroke 3.3%, peripheral embolization 3.3%, myocardial infarction 6.5%, vascular access site repair 9.8%, and transfusion 30.8%. These events will contribute to the overall morbidity of percutaneous aortic valve replacement. It is likely that surgical repair or the use of Perclose (Abbott Laboratories, Abbott Park, IL) devices to pre-close the access site will reduce vascular complications and the need for transfusion. Lastly, it is important to remember that transseptal puncture, which is performed during the antegrade technique, is associated with a 1 to 2% risk of cardiac perforation and tamponade.
Antegrade approach
Currently there are two approaches used for implantation for the Cribier-Edwards PHV, the antegrade and the retrograde techniques (Figure 1).
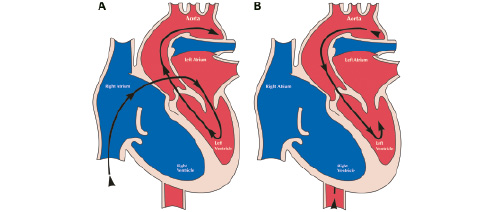
Figure 1. Techniques for percutaneous aortic valve replacement. Antegrade approach (A) and retrograde approach (B).
At this time more PHVs have been implanted via the antegrade technique, although experience is being rapidly gained utilizing the retrograde technique. The antegrade approach is a technically challenging and complex procedure. Femoral venous access is obtained and a transseptal puncture is performed. A balloon flotation catheter is used to cross the mitral valve, is looped in the left ventricle and then advanced across the aortic valve. Ultimately, a super stiff guide wire is advanced into the descending aorta, snared and externalized via the femoral artery. A critical part of the procedure is to maintain the loop formed in the left ventricle. If the loop is shortened the anterior mitral leaflet may be tethered and constrained leading to torrential mitral regurgitation (Figure 2).
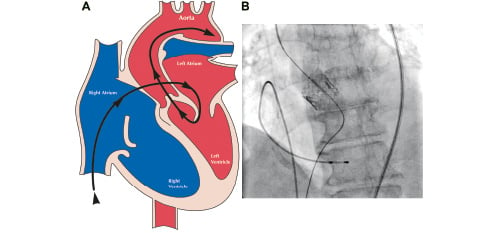
Figure 2. Shortened guidewire loop in left ventricle constraining the anterior mitral leaflet (A) and fluoroscopic correlate (B).
Acute severe mitral regurgitation is poorly tolerated in these fragile patients and leads to rapid decompensation. This complication has occurred in several patients resulting in haemodynamic collapse. To minimize the risk of loop shortening, it is helpful to advance a Sones catheter retrograde into the left ventricle. This maneuver has greatly helped maintain the loop configuration (Figure 3).
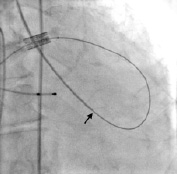
Figure 3. Sones catheter advanced retrograde into the left ventricle to maintain guidewire loop (arrow).
If loop shortening occurs, it is essential to expeditiously reform the loop. This can be accomplished by advancing a Sones catheter into the left ventricle and then pushing the wire from venous and arterial sites.
A second, but related, complication involves trauma to the anterior mitral leaflet. If the bare guidewire is pulled across the mitral leaflet, laceration of the leaflet may occur. This can induce permanent severe mitral regurgitation (Figure 4).
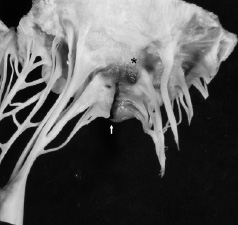
Figure 4. Anterior mitral leaflet. Guidewire induced laceration (arrow) and haemorrhage (asterisk).
This complication occurred twice during early experience resulting in death due to cardiogenic shock. To prevent laceration of the mitral leaflet it is essential to keep the guidewire insulated with catheters at anytime the wire is manipulated. Typically we use a 6 French pigtail catheter to insulate the wire across the mitral valve. This catheter is kept in place until the PHV is inserted. When implantation is complete the pigtail is re-advanced before the wire is removed. Since this technique has been adopted, no further mitral leaflet lacerations have occurred.
Retrograde approach
Ultimately we believe that the retrograde approach will be the preferred delivery method in the vast majority of patients. The retrograde technique is attractive for several reasons. First, it is less technically demanding and similar to retrograde balloon aortic valvuloplasty. Second, it avoids the potential mitral leaflet complications. Third, transseptal puncture is not necessary. Retrograde implantation was first performed at our institution as a bail out after attempted antegrade implantation was aborted due to mitral leaflet injury.9 Several subsequent attempts at other institutions were unsuccessful due to poor delivery catheter pushability and guidewire bias into the commissure rather than the central orifice (Figure 5).
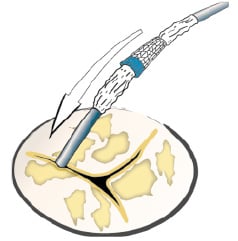
Figure 5. Aortic valve with commissural wire bias.
These pitfalls have been surmounted by the development of the Flex Catheter by Edwards Lifesciences, which John Webb has used in over 25 patients to date (Figure 6).
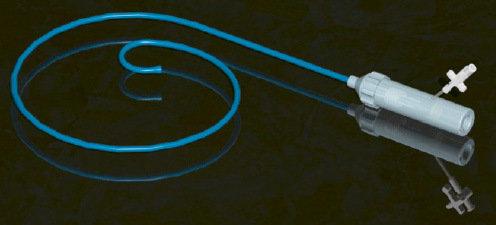
Figure 6. Flex Catheter.
This deflectable catheter allows coaxial alignment of the PHV and greater pushability and therefore consistent delivery of the PHV to the intended implant site.
Currently, 22 and 24 French systems are available for the 23 mm and 26 mm PHVs, respectively. Because of the large profiles this implant option may be limited in patients with small, stenotic, calcified, or tortuous iliofemoral systems. In addition to access site bleeding, these patients are likely at higher risk of vessel trauma or transsection, peripheral atheroembolism, and nondeliverability. In general, patients with common femoral and iliac artery diameters of less than 8 mm are currently excluded for fear of vessel transsection or severe dissection. However, patients with overall adequate iliofemoral size but focal moderate stenoses have been treated via the retrograde approach without complication. Because the sheath is not only large in diameter but also stiff, patients with significant iliofemoral calcification or tortuosity should be excluded since they may be at risk for vascular damage, including rupture. Currently most sites are achieving vascular access percutaneously and then closing the site surgically. Theoretically, the access site could be “pre-closed” with two 10 F Perclose devices. Some centres routinely use this technique in the setting of endovascular aneurysm repair with good anecdotal results. As greater experience is gained with the retrograde approach, it may be reasonable to try this technique. One caveat is that there is a significant learning curve to gain facility with the older generation 10 F Perclose device. For these reasons, patient screening and selection is of utmost importance in order to avoid vascular injury and poor patient outcomes. It is mandatory that device profile be reduced to minimize these complications. Much needs to be learned regarding anatomic features of the iliofemoral system which are associated with technical success and, conversely, complications and failure.
Shared complications
While the antegrade approach is limited mainly by technical complexity and mitral leaflet injury and the retrograde approach is limited by access site complications there are several important complications shared by both techniques. These primarily include PHV embolization and paravalvular aortic regurgitation but conceivably may also include obstruction of the coronary ostia.
Embolization of the PHV can occur as forward cardiac flow ejects the delivery balloon/PHV unit during balloon inflation (Figure 7).
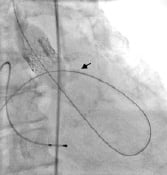
Figure 7. Embolized percutaneous heart valve (arrow denotes aortic cusp calcification).
This complication has occurred with both the antegrade and retrograde approaches. Likely the greatest factor contributing to embolization is forceful ventricular contractility against the inflated delivery balloon. Other possible factors include annulus/PHV mismatch and high positioning of the PHV. The most critical strategy to reduce the risk of embolization is the induction of cardiac standstill via rapid right ventricular pacing (Figure 8).
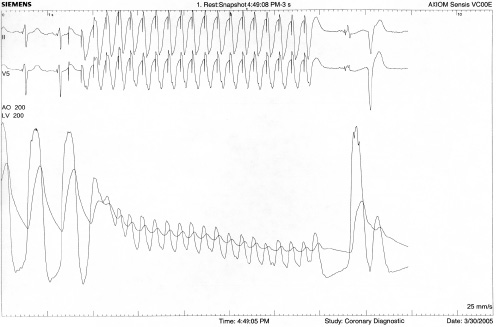
Figure 8. Reduction in blood pressure with rapid ventricular pacing.
Initially pacing is tested at 220 beats per minute and if exit block occurs the rate is reduced –usually to a rate no lower than 180 beats per minute– until effective capture results. Balloon inflation must begin only after rapid pacing reduces systolic blood pressure to less than 30 mmHg or 40 mmHg, and rapid pacing must continue until after the balloon is fully deflated. Otherwise, a single strong cardiac contraction could dislodge the PHV before the delivery balloon is deflated. Other important steps to avoid embolization include: proper placement of the PHV (placing the middle of the PHV at or just below the aortic leaflet calcification; high placement may increase the risk of embolization), accurate annular sizing (large annular size may not adequately hold the PHV in place), and avoiding any tension on the delivery balloon during implantation. If embolization does occur, the PHV may be implanted in the aortic arch or descending aorta and if necessary may be stented open with a tracheal stent. Importantly all embolizations have occurred during implantation and no late migrations have been noted.
Paravalvular aortic regurgitation is possibly the most important complication seen after PHV implantation. Mild to moderate aortic regurgitation seems to be well tolerated in these patients and is likely a worthwhile trade-off for critical aortic stenosis. On the other hand, acute severe aortic regurgitation into hypertrophied, noncompliant ventricles is not well tolerated and may cause persistent or even worsening heart failure. Some degree of aortic regurgitation is seen in most patients while 3+ to 4+ regurgitation may occur in up to one third of cases. Heavy calcification of the aortic cusps may prevent full apposition of the PHV stent at the native commissures. This gap is the substrate for paravalvular aortic regurgitation (Figure 9).
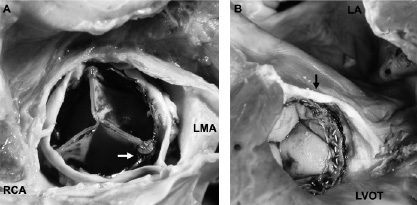
Figure 9. Percutaneous aortic valve. A. Superior view of the PHV. The right coronary (RCA) and left main coronary (LMA) ostia are above the PHV. Free space (arrow) between the PHV and native commissure as cause of paravalvular aortic regurgitation. B. Inferior view of the PHV. Left ventricular outflow tract (LVOT); left atrium (LA); and PHV above the base of the anterior mitral valve leaflet (arrow).
Reduction of paravalvular aortic regurgitation is of paramount importance. Likely, appropriate PHV sizing will contribute to this end. Preliminary experience from John Webb’s centre suggests that the larger 26 mm PHV reduces the degree of paravalvular aortic regurgitation. Much needs to be learned about aortic annular sizing including which noninvasive modality is most accurate and the optimal device size for any given aortic annulus diameter. Hopefully this, coupled with refinements in device design, will reduce the incidence and degree of paravalvular aortic regurgitation.
Other potential complications include obstruction of the coronary ostia by the PHV fabric or calcified native aortic cusp, haemolysis, accelerated leaflet degeneration, and thrombus formation on the stent struts. Scrupulous patient follow-up is essential to determine whether these or other as yet unforeseen complications arise.
Conclusions
Percutaneous aortic valve replacement is an exciting novel investigational therapeutic for the treatment of nonoperative patients with severe aortic stenosis. Many patients have already experienced impressive haemodynamic results and dramatic clinical improvement. However, others have suffered devastating complications as described above. It is not surprising that such an innovative device and technique would encounter hurdles in early clinical trials, particularly since the patients targeted for treatment are quite ill with multiple comorbidities and little physiologic reserve. It is remarkable that in a brief period of time these complications have been identified and great strides have been made to minimize them. Undoubtedly this nascent technology will undergo numerous iterations in response to these and other as yet unforeseen complications. Only by continued rigorous scientific study and scrupulously honest and transparent assessment of the weaknesses, limitations, and complications of this technology will these difficulties ultimately be surmounted and percutaneous aortic valve replacement will emerge as a safe and effective treatment option for high-risk patients with severe aortic stenosis.

