Abstract
Reperfusion and medical therapies are able to restore blood flow and to improve symptoms, functional class and in some cases increase survival. Cell therapy may be a potentially attractive approach to restore myocardial contractile performance after an infarction injury.
In the presence of fibrotic post-infarction scar with no detectable myocardial viability, direct myocyte precursors, i.e. myoblasts, are one of the first and still most encouraging cell types used in cell-based therapy, being considered as a potential source of new muscle fibres.
Direct intramyocardial myoblast injection during open-chest surgery or as a stand-alone percutaneous procedure has been shown to be feasible and safe in several animal and human clinical studies.
The present review explores the current clinical experience with autologous skeletal myoblasts transplantation in patients with post-infarction heart failure.
Introduction
Heart failure is rapidly becoming a major worldwide epidemic and, in western countries, is by far the most common cause of hospitalisation. The increasing prevalence of heart failure directly relates to increasing age1, a significant reduction in sudden cardiac death due to rapid revascularisation, the use of internal cardio-defibrillators (ICDs)2 and improved long-term survival of patients with acute myocardial infarction (AMI).
Myocardial necrosis, and the subsequent formation of fibrotic scar which replaces viable myocardium, leads to depressed systolic function, reduced diastolic compliance, left ventricular remodelling, aneurysm formation and ultimately to the progression of congestive heart failure.
Revascularisation therapies have been able to restore perfusion and contractile function to post-infarct stunned or hibernated myocardium. Chronic medical therapies are further able to improve symptoms, functional class and, in some cases, increase survival, however these medical interventions are not capable of replacing or repairing damaged myocardial tissue. The potential to repair and grow new tissue within the necrotic scar as a result of cell transplantation has thus drawn widespread attention, resulting in several clinical studies sharing the common purpose of regenerating or repairing myocardium by the delivery of new contractile or pluripotent cells3-10.
One of the first and still most encouraging cell types used in cell-based therapy are autologous skeletal muscle stem cells (also referred to as myoblasts). Skeletal myoblasts are mononuclear progenitor cells usually residing in a quiescent state between the basal membranes of skeletal muscle fibres. Following muscle injury, recruited non-contractile myoblasts proliferate and differentiate into functional (contractile) skeletal myocytes, ultimately fusing into multinucleated myotubes. When transplanted intra-miocardially, myoblasts have been shown to differentiate into myotubes11-13 and contract synchronously with host cardiomyocytes, even in the absence of gap junctions.
The literature suggests that skeletal myotubes, in fact, are not capable of expressing the trans-membrane protein connexin 43 (Figure 1), a necessary element in achieving mechanical and electrical coupling with myocardial tissue, and thus do not couple with resident cardiomyocytes14,15.
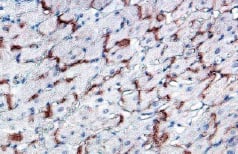
Figure 1. Formalin fixed paraffin embedded rat heart stained with Connexin 43 using LSAB-DAB. Note membrane staining pattern.
Nonetheless, myoblasts have been shown to actively contribute to systolic contraction. It was hypothesised that stretching signals and direct ionic trans-membrane currents12,16 may enhance the contractile function within the area of fibrous post-infarction scar tissue with a lasting positive effect on global systolic function17,18. In addition to the aforementioned mechanical effects, myoblasts may improve cardiac function also by paracrine mechanisms. These cells are capable of secreting vascular endothelial growing factor (VEGF) and insulin growth factor I (IGF-1) in concentrations which can mobilise resident quiescent cardiac cells and promote angiogenesis19, 20. Moreover, myoblast engraftment is associated with a marked attenuation of matrix metalloproteinase-2 and -9 up-regulation. This, together with VEGF and IGF-1 release, may protect peri-infarction tissues against fibrotic remodelling21.
Cell therapies can be delivered via intracoronary injection, percutaneously by direct injection in the ventricular wall or with an open chest approach. While some cell types including bone marrow-derived and blood-derived endothelial progenitor cells22 are capable of extravasating and migrating to ischaemic areas, skeletal myoblasts are not, making intracoronary delivery infeasible and even potentially harmful due to potential obstruction of the microcirculation and possible embolic micro-infarctions. In contrast, direct myoblast injection during open-heart surgery or as a stand alone percutaneous procedure has been shown to allow unrestricted cell uptake from the circulation without embolic risk in several animal and human clinical studies8,23-25.
Cell transplantation during open heart surgery
The initial clinical experience with myoblast transplantation was achieved using trans-epicardial delivery during open-chest CABG surgery on patients with severely reduced LVEF26-31. This approach showed certain advantages, including easy access to the target area and delivery of a sufficient amount of cells per injection. Results from this early experience were encouraging. After the first case report on autologous skeletal myoblast transplantation was published in Lancet in 2001 by P. Menasché28, two phase-I studies on the safety and efficacy of combined myoblast transplantation and CABG were started in Europe – Paris and Poznan – in 2002 and 2003, respectively24-26. Both studies showed the procedure to be safe and effective, providing a significant improvement in regional wall motion (by TTE) and global LVEF, as well as an increase in viability by positron emission tomography (PET) and a significant improvement in symptoms. These encouraging results were confirmed in the same year by Herreros and colleagues27 in a population of twelve patients with previous MI and manifest ischaemic coronary artery disease. At 3 months, both regional and global LV function were increased (LV improved from 35.5±2.3% at baseline to 53.5±5% at 3 months), with viability of the treated cardiac tissue in the area of prior infarct area demonstrating improvement by PET. No cardiac arrhythmias were detected during the follow-up period.
Long-term follow-up results from a multicentre, dose-escalating safety study of myoblast transplantation during CABG (24 patients) or LVAD implantation (6 patients) was also published in 2005 by Dib and colleagues31. Magnetic resonance imaging (MRI) scans, as well as echocardiographic and PET evaluations, showed increased viability of the grafted scar and an improvement in the mean LVEF from 28% to 35% and 36%, after 1 and 2-year follow up, respectively. Further, histological analyses of hearts from patients undergoing LVAD treatment and subsequent heart transplantation, demonstrated survival and engraftment of skeletal myoblasts within the infarcted areas of the myocardium.
At the 2006 AHA meeting, Menaché and colleagues presented the six month follow-up of the MAGIC trial, a multi-centre, randomised, placebo-controlled trial designed to assess the safety and efficacy of two doses of myoblasts (high dose: 8x106 cells and low dose: 4x106 cells) injected concomitantly with CABG. The original study design called for 300 patients, however the trial was suspended following an interim analysis of the first 120 patients, as the study was not expected to meet its primary efficacy endpoint (regional and global wall motion improvement at six months by echo analysis). It is noteworthy, however, that evidence of positive remodelling presented in the 8x106 cell group, as LVEDV, LVESV and LVEF improved significantly vs. control as measured by MUGA. Further, both the incidence of MACE and ventricular arrhythmias did not significantly differ between the treated and the control group, suggesting that the procedure is safe and not associated with an increased risk of arrhythmic events or sudden death. Though data deriving from the aforementioned trials are encouraging, many eligible patients who could potentially benefit from myoblast transplantation are not candidates for epicardial surgical delivery due to their clinical condition and the risks associated with surgery and general anaesthesia. Moreover, proper interpretation of the clinical outcomes obtained in these cell therapy studies is difficult because of the confounding and beneficial effect of the concomitant revascularisation procedure. Thus, a minimally invasive, catheter-based delivery approach is highly desirable. A percutaneous approach with cell injection as a stand alone procedure allows for targeting areas inaccessible by surgery (e.g. septum), treating high-risk patients and performing repeat injections with minimal risk. More importantly from a scientific perspective, percutaneous cell transplantation allows for the evaluation of intra myocardial myoblast implantation without confounding factors.
Endo-ventricular approach
Myoblasts delivery via endo-ventricular catheters allow direct cell injection into the targeted area of the injured left ventricular wall. Catheter-based trans-endocardial injection is performed using a needle catheter advanced across the aortic valve and positioned perpendicularly against the ventricular wall under fluoroscopic guidance or, in some cases, using an electromechanical mapping of the endocardial surface (NOGA) which is capable of defining viable and scarred myocardium (Figure 2)8,32,33. In 2003 the Rotterdam group8 employed this technique to inject autologous myoblast suspensions into the area of post-infarction injury of five patients. This early experience, the first to document the feasibility of this particular approach, showed a significant increase in LV ejection fraction (LVEF) and regional wall motion at three months follow-up by angiography, as well as a trend towards increased LVEF as observed by angiography and nuclear scan at the six month follow-up.
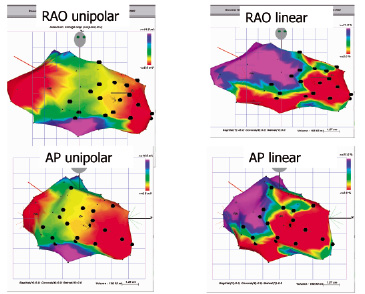
Figure 2. Unipolar (left) and linear (right) NOGA maps acquired during cell delivery procedure. The myocardial scar is scaled red indicating an area with a < 6 mV voltage and a < 2% shortening. Black dots indicate the trans-endocardial injection sites.
A sub-study conducted to evaluate the efficacy of myoblast transplantation on regional and global LV functional by dobutamine stress echocardiography with dobutamine infusion and tissue doppler imaging (TDI), likewise showed an improvement in the target wall systolic velocity and global LV function during low dose dobutamine infusion, indicating an improvement in the contractile reserve34. More impressively, these haemodynamic improvements were shown to persist one year after percutaneous myoblasts transplantation: pressure-volume loop analysis confirmed both systolic and the diastolic functional recovery (Figure 3)35.
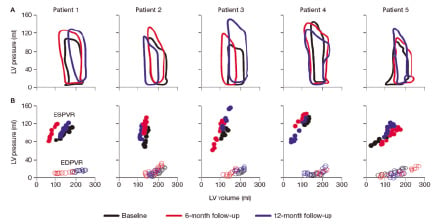
Figure 3. Steady-state pressure-volume loops (A), end-systolic pressure-volume relationship (ESPVR) and end-diastolic pressure-volume relationship (EDPVR) (B), for each patient. LV: left ventricular.
In 2004, Ince and colleagues published the results of a case control study in which myoblasts were injected in six post-AMI patients with severe chronic LV dysfunction. At 12 month follow up, LVEF of treated patients rose from 24±7% at baseline to 32±10% with a significant improvement of NYHA functional class, while in matched control patients LVEF decreased from 25±5% to 21±4% (p value <0,05) and NYHA functional class remained unchanged23. Similar results, though not as significantly pronounced, were observed by Serruys et al36 in the first multicentre study (n=15) designed to assess the safety and feasibility of trans-endocardial myoblast injections. At 12 months follow-up, both regional wall motion scores and NYHA functional class were significantly improved while LVEF increased from 34±10% to 37±10% (p=0.26).
The safety and efficacy of skeletal myoblast transplantation using the Myocath® delivery system (Bioheart, Inc., Sunrise, FL, USA) is currently under investigation in two multicentre clinical trials ongoing in Europe (SEISMIC, phase II trial) and the US (MYOHEART, phase I trial). The SEISMIC trial will enrol 46 patients, randomised 2:1 to the Myocell™ treatment arm (n=30) versus optimised medical therapy controls (n=16). All patients will be analysed for six months. Recruitment for the MYOHEART study, a non-randomised trial, was recently completed. All patients will be analysed for 12 months, with the expected study completion date being November 2007. In each of the aforementioned studies, interim analysis data evaluated for DSMB review suggest positive trends towards improvement in treated patients especially respective to patient quality of life, though at this time these are statistically non-significant observations.
Although shown to be feasible and safe, cell delivery by percutaneous based injection requires further optimisation. It is challenging to consistently achieve full and stable apposition with the ventricular wall with most endo-ventricular catheter systems currently available as injection pressures can push back the needle tip from the myocardium, potentially allowing a back flush of cells from the puncture site to occur. In addition, following deployment, needle positioning against the endocardial surface does not synchronously follow the natural heart movement, which makes injection into thin post-infarction scars or in the border zone of the infarct difficult.
In order to minimise these technical issues and to make the procedure easier, safer and more effective, a new generation of transendocardial delivery systems are being designed. The most prominent change consists of a different needle tip design: the traditional end hole needle has been replaced by a closed tip needle with side-port holes (e.g. Myocath™, Bioheart Inc.) in order to spread the cell injected over a larger target area, rather than relying solely on perpendicularly delivery to a single spot (Figure 4). This release pattern could diminish the risk of perforation and improve catheter stability with more reliable wall apposition by decreasing injection pressures and back flush.
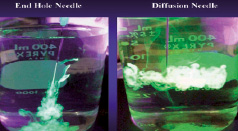
Figure 4. Old Myocath™ catheter with a single end hole needle (left) and the new Myocath™ delivery system with side port holes (right).
Trans-venous approach
Catheter based cell infusion through coronary veins is a relatively novel approach.
The injection catheter, residing within a coronary vein and moving together with the heart wall, provides stability to the delivery system and allows an accurate and effective cell delivery, while minimising the possibility of cell loss and wall perforation.
So far, the most compelling results using the trans-venous injection delivery in humans have been achieved by Siminiak and co- workers24,25 in the POZNAN trial25, a phase one clinical trial designed to assess safety and feasibility of autologous myoblast transplantation performed by the TransAccess® catheter, a composite catheter system combining a phased array IVUS and a pre-shaped extendable 24 gauge nitinol needle.
The TransAccess system is placed in the target coronary vein (Figure 5), the intravascular orientation is performed using the corresponding artery, pericardium, and ventricular myocardium as landmarks with IVUS imaging (Figure 6). After verification of the proper TransAccess catheter position, the nitinol needle is extended into the myocardium and the injection catheter (MicroLume™) is advanced through the needle deep into the myocardial scar area, to allow myoblast suspension injection.
The use of the anterior interventricular vein and the middle cardiac vein, parallel to the posterior descending coronary artery, both were shown to be feasible. Two to four intramyocardial injections were performed in each patient, delivering up to 100 million cells in 0.6 - 2.5 ml of saline solution. The procedure was reported to be technically successful in nine out of 10 patients and did not cause any periprocedural adverse event. At six months follow-up, NYHA class improved in all patients and LVEF, assessed by echocardiography, significantly increased by three to eight percentage points in six out of nine patients, confirming both the safety and feasibility of trans-venous intramyocardial myoblast injections by the TransAccess® system.
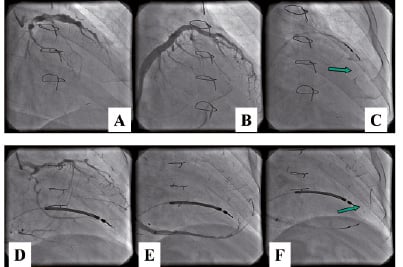
Figure 5. Trans-coronary-venous myoblast transplantation using anterior interventricular vein (A,B,C) and middle cardiac vein (D,E,F). A,C: coronary angiography; B,D: coronary venography, C,F: Trans Access® catheter system positioned within vein - arrow indicates tip of the microcatheter during cell injection.
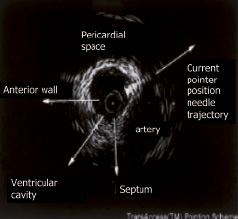
Figure 6. TransAccess® system orientation by IVUS imaging. The needle is oriented taking the corresponding artery, the pericardium, and the ventricular myocardium as landmarks.
Safety issues
While ex vivo experiments suggest the possibility of cell fusion and the formation of a synchronous beating network in a co-culture of myoblasts and cardiomyocytes37,38, several studies have shown skeletal myotubes to be incapable of expressing connexin 43 (gap junction protein) and physically coupling with host cardiomyocytes, and from beating in synchrony with the rest of the heart12,39,40.
In the absence of this electromechanical coupling, the arrhythmogenic mechanisms remain unclear. Myoblasts have been shown to generate burst of action potentials, which may induce ventricular extrasystoles and tachycardias through electro-tonic interactions41. Moreover, an arrhythmogenic role could be related to the injection procedure itself, including myocardial puncture, inflammatory response to injured transplanted myoblasts (paracrine effect) and immune reactions41. Major ventricular arrhythmias were observed in the majority of humans studies conducted thus far, irrespective of the delivery route or cell dose8,25,26,28,31.
In the first clinical series published by Menasché at al26, four patients who underwent autologous skeletal myoblast transplantations during CABG experienced sustained episodes of ventricular tachycardia (VT). Similarly, the possible arrhythmogenic effect has also been observed in studies using an endo-ventricular catheter approach. In the study conducted by Smits et al42, one patient experienced sustained VT at six weeks and subsequently received subsequently an ICD, while more seriously, two sudden deaths occurred. These events prompted DSMB consultation and the recommendation for all subsequent patients to receive prophylactic ICD implantation. Interim results from the MAGIC trial presented at the AHA 2006 scientific session, however, suggested no statistically significant difference between the treatment and placebo groups in terms of ventricular arrhythmias. The interim analysis of the SEISMIC study suggests similar results. It should be emphasised that patients enrolled in these initial studies did not receive prophylactic antiarrhythmic therapy even though the target population of advanced ischaemic chronic heart failure easily develops ventricular arrhythmic events through the natural progression of the disease. Statistical analysis indicates that the risk of arrhythmic events is largely restricted to the first 30 days following the procedure, and recent clinical data suggest that prophylactic ICD implantation and use of Class III anti-arrhythmic medication may largely control risk of arrhythmias immediately following cell implantation.
Amiodarone was administered prophylactically to all patients in the POZNAN trial, and VT episodes occurred only in a single patient who was not compliant with amiodarone use during the first few weeks following the procedure25. In fact, it could be suggested that myoblast related VT may be circumvented by concomitant amiodarone therapy, thereby obviating an AICD implantation.
In sum, though several clinical trials in a small number of patients initially suggested a potential risk of ventricular arrhythmia following myoblast transplantation, it remains difficult to ascertain whether skeletal myoblasts are indeed arrhythmogenic as well as to unveil the underlying mechanism. Because the majority of episodes of ventricular arrhythmias occurred early after transplantation, it is plausible that their occurrence may be related to the procedure itself (due to the intramyocardial puncture or inflammatory reaction) rather than to the lack of gap junctions between the graft and host myocardium, which would result in later term arrhythmic events.
Conclusions
Although cell therapy for human cardiac regeneration is still an experimental field and only taking its first steps in clinical practice, a considerable number of preclinical animal studies and phase I human studies have been completed over the past years. These trials have shown that autologous myoblast transplantation, both during cardiac surgery and by percutaneous injection, is feasible, safe and appears to hold significant promise. However, the cell delivery still needs to be improved and the occurrence of arrhythmic complications needs to be observed carefully, with prophylactic attempts made to minimise their occurrence during the initial days following transplantation. Several large phase I/II clinical studies are currently under way in both Europe and the USA (e.g. SEISMIC and MYOHEART II) in order to assess the real potential arrhythmogenic effect as well as the efficacy of myoblast transplantation in chronic post-infarction myocardial injury. The final results from these trials will be available within the next year and will provide useful insight as to the possible future role of autologous myoblast therapy in the treatment of chronic heart failure.

