Abstract
Aims: To assess plaque characteristics by multi-detector computed tomography angiography in patients with known coronary artery disease and to compare these findings with those obtained with intravascular ultrasound-derived radiofrequency analysis of plaque composition.
Methods and results: By computed tomography, lesions were classified on the basis of Hounsfield Units as non-calcified, calcified, or mixed. By intravascular ultrasound-derived radiofrequency analysis, plaques were classified according to the relative composition of components with specific backscatter characteristics (necrotic core, fibrous, fibro-fatty, calcium). Thin cap fibroatheroma (defined as necrotic core component >10% without evidence of fibrotic cap, calcium >5%, remodelling index >1.05) was considered as vulnerable plaque. Seventy-eight plaques were analysed. By computed tomography, 22 plaques were classified as non-calcified and 56 as mixed. A higher incidence of mixed plaques was observed among lesions causing unstable angina and non-ST elevation myocardial infarction compared to stable angina (76% vs 38%, p=0.04). Plaque composition by radiofrequency analysis was significantly different between mixed and non-calcified plaques by computed tomography. The calcium content was 6.0±3.2% vs 2.5±1.8% (p=0.001), necrotic core was 14.0±6.9% vs 7.5±5.6% (p=0.03) and fibrous tissue was 59.0±7.5% vs 67.0±5.9% (p=0.03), for mixed vs non-calcified plaques, respectively. Positive, negative predictive value and diagnostic accuracy for detection of vulnerable plaque by computed tomography was 77, 54 and 59%.
Conclusions: Mixed plaque by computed tomography correlates with plaque composition as determined by intravascular ultrasound-derived radiofrequency analysis. However, the present diagnostic accuracy of computed tomography is not high enough to support its use for non invasive detection of vulnerable coronary plaque.
Introduction
Determination of plaque composition in patients with coronary artery disease (CAD) may become an important tool for the identification of plaques at higher risk of causing sudden cardiac death and unheralded myocardial infarction1. Although such strategy has not yet been proven effective, healthy carriers of high-risk plaques could potentially benefit from targeted interventional and/or medical therapies. Atherosclerotic lesions are a complex mix of various tissue subtypes and it is well known that plaque composition is related to plaque activity2. Recently, 64 multi-detector computed tomography coronary angiography (MD-CTA) has been validated as a non invasive method for detection of coronary atherosclerosis3. A few studies have attempted to classify plaque composition on the basis of density scores using Hounsfield units (HU).4,5 These studies have demonstrated that MD-CTA density measurements show a strong correlation with lesion echogenicity on grey scale intravascular ultrasound imaging (IVUS). However, for accurate characterisation of lesions with low echogenicity, which can have either high lipid content or mixed composition, grey scale IVUS is poorly accurate.6 A new technique that uses spectral analysis of the radiofrequency ultrasound backscatter signals, known as intravascular ultrasound virtual histology (VH IVUS), was recently validated both in vitro and in vivo allowing thus a detailed in vivo assessment of plaque composition.7,8 Predictive accuracies of 93.4% in fibrous, 94.6% in fibro-fatty, 96.8% in calcified and 95.1% in necrotic core regions have been found compared to histopathology.9 This allows the identification of VH IVUS-derived thin-cap fibroatheroma (VH IVUS-TCFA), the plaque type that shows the highest association with plaque rupture when assessed by post-mortem histopathology.10 TCFA is composed of lipid-rich atheromatous core, a thin fibrous cap with macrophage and lipid infiltration, decreased smooth muscle cell content and expansive remodelling.11,12 Data correlating plaque characterisation by MD-CTA with the corresponding VH IVUS analysis of plaque composition are presently unavailable. This study was undertaken to correlate plaque type as determined by non-invasive MD-CTA to the tissue and plaque characterisation obtained by invasive VH IVUS radio frequency analysis in patients with known coronary artery disease and various clinical presentations. The aim was to evaluate whether MD-CTA can predict plaque type on the basis of densities, and specifically whether MD-CTA can be used as a tool to detect plaque features associated with the presence of VH IVUS-TCFA.
Methods
Twenty-eight patients with known coronary artery disease undergoing both MD-CTA and invasive coronary angiography were included in this study. Categorisation of clinical presentation was based on all available data in order to classify patients as acute coronary syndromes (ACS), either non-ST elevation myocardial infarction (NSTEMI), ACS without increase in troponin level (UA) or stable angina. The local Medical Ethics Committee of both institutions approved the study protocol and all patients gave informed consent.
MD-CTA angiography
Scanning was performed either with a Sensation 64 scanner (Siemens Medical Solutions, Forchheim, Germany) or 64-slice MDCT (Aquilion 64, Toshiba Medical Systems, Japan). Scanning parameters were 64-x0.6-mm collimation, 330-ms rotation time, 3.8 mm/rotation table feed, 120-kV tube voltage, and 850-mAs tube current for the Siemens Scanner and 64-x0.5-mm collimation, 400-ms rotation time, 135-kV tube voltage, and 350-mAs tube current for the Toshiba scanner. A bolus (on average 90 ml at 5 ml/sec) of iodinated contrast material (Iomeron 400, 816.5 mgI/ml) was administered followed by a 50 cc saline flush. Patients with a baseline heart rate >65 beats/min were given beta-blockers (metoprolol 25 to 100 mg orally prior to examination, supplemented by intravenous administration as required). All the datasets were evaluated off-line on an image analysis workstation (TeraRecon Inc, San Mateo, CA, USA) by two independent experienced observers (ID, PV). MD-CTA data were evaluated on 2D axial, maximum intensity projections (MIP) and curved multiplanar reconstructions (cMPR). Plaque severity was assessed visually and lesions were designated as non-obstructive (≤50% luminal narrowing) or obstructive (>50% luminal narrowing). Plaques were classified on the basis of density in three groups as previously described.13,14 For the purpose of visual sampling of the attenuation of the lesions, colour mapping was used, assigning colours to different densities on longitudinal and axial MPR images (Figure 1).
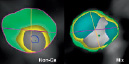
Figure 1. Colour coding maps on axial images of a non-calcified (left) and a mixed plaque (right). Colours are semi-automatically assigned to the lesions. Pixels with a density value between 150HU and 0HU are yellow, pixels with a density value between 1HU and 130HU are light green, pixels with a density value of more than 130HU are dark green. The residual lumen is not colored.
The densities measured needed to be confirmed in at least two planes and were made in the plaque subtypes that were assigned different colours, with a region of interest of 5 pixels. These measurements were repeated at least twice in each “colour zone”. Group 1, non-calcified lesions, if no dense calcium (higher attenuation than the opacified lumen) could be visualised, and if the maximal densities found in the lesion were lower than 130 HU. Group 2, calcified lesions, if the plaque displayed high-density calcium (higher than the opacified lumen) and no associated low-density components (<130 HU) surrounding or adjacent to the calcified zone could be detected. Group 3, mixed, whenever the lesion showed characteristics of group one and two combined. The threshold of 130 HU was chosen as the lower threshold for calcification, since this is the value used for determination of Agatston calcium scoring15, and since this was the highest value that non-calcified plaques showed in an in vitro study of plaque attenuation.16
The positive and negative predictive values and the diagnostic accuracy for vulnerable plaque identification by MD-CTA, using VH-IVUS as gold standard, were computed. The true positive rate for vulnerable plaque was calculated as the proportion of mixed plaques by MD-CTA characterised as VH IVUS -TCFA.
Invasive coronary angiography and VH IVUS imaging
Coronary angiography was performed according to local practice most frequently via the femoral route. Multiple projections were analysed after intracoronary nitrate injection. VH IVUS was performed with a 20 MHz catheter (2.9 Fr, Volcano Therapeutics, Rancho Cordova, CA, USA) at 0.5 mm/sec automatic pullback rate. The data were analysed offline using the pcVH 2.1 and qVH software (computer-based qualitative and quantitative analysis software, Volcano Corp.) by two operators blinded to the MD-CTA plaque composition results. In case of disagreement that could not be resolved, the data were reviewed and consensus was reached together with a third independent observer. The presence of fresh thrombus was carefully screened at the time of contour detection based on known grey scale features including partial detachment from vessel wall and/or irregular luminal borders.
Details regarding the extensive validation of the technique, including explanted human coronary arteries, have been previously reported.7 Briefly, VH IVUS uses spectral analysis of ultrasound radiofrequency backscatter data to build specific tissue maps that are correlated with a given spectrum of the radiofrequency signals and assigned colour codes (fibrous: labelled green, fibro-fatty: labelled greenish-yellow, necrotic core: labelled red, calcium: labelled white).
For this study, the analysis plan was as follows:
– per lesion analysis, in which the regions of interest on the coronary tree were identified by MD-CTA. To ensure that exactly the same coronary segments were compared with both imaging modalities, fiduciary points such as side branches were used as well as the distance from the coronary ostium, as proposed previously for serial IVUS investigations.14
– per three-frames analysis, involving the frame within the analysed segment with the minimal lumen area (MLA) plus one adjacent proximal and distal frame (150 microns in thickness each).
In the per lesion analysis, the tissue characterisation of the plaque, described as percentage of plaque volume (% fibrous tissue,% fibro-fatty,% calcium,% necrotic core), its extension (plaque burden) and the remodelling index (RI) were computed. Remodelling index was calculated as follows: (lesion EEM / mean reference EEM area).
In the three-frames analysis, the morphological classification of the plaque was performed according to the modified American Heart Association histological classification by Virmani et al9 adapted for VH IVUS plaque classification based on plaque composition and location of different tissue components, as listed below:
– Pathological intimal thickening (PIT): mixture of fibrous/fibro-fatty >5%, calcium <5%, necrotic core<5%.
– Fibroatheroma (FA): fibrotic cap and significant confluent necrotic core >5% in fibrous and/or fibro-fatty tissue.
– VH IVUS thin cap fibroatheroma (VH IVUS-TCFA): necrotic core component >10% confluent to the lumen without evidence of fibrotic cap, calcium >5%, remodelling index >1.05.
– Fibrocalcific plaque: mainly fibrous plaques, with dense calcium >5%, necrotic core <5%.
Statistical analysis
Continuous variables are presented as mean±SD. Categorical data were expressed as counts and percentages. Continuous variables were compared by 2-tailed student t test or by one-way analysis of variance (ANOVA). The Pearson χ test, or Fisher’s exact test for sparse data, were used for comparing frequency of occurrence. Quantitative IVUS and MD-CTA data were correlated by means of the Pearson Correlation coefficient. The inter-observer agreement for the lesion classification in calcified, mixed or soft plaque by MD-CTA was tested by Cohen’s Kappa statistics. A probability value of <0.05 was considered significant.
Results
Patient characteristics are displayed in Table 1.
The mean age was 57±12 years. Of note, twenty-three patients were male (82%) and the prevalence of diabetes was low at 7%.
Seventy-eight lesions were analysed by MD-CTA and VH IVUS. Plaque was located in the LAD in 54%, the LCx in 22%, and the RCA in 24%. Coronary plaque was obstructive in 23% and non-obstructive in 77%.
With MD-CTA, 56 lesions were defined as mixed (Figure 2) and 22 as non-calcified plaques (Figure 3).
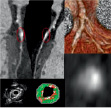
Figure 2. Orthogonal curved multiplanar reconstructions (top left), axial image (bottom right) and volume rendered image (top right) of a mixed plaque in the LAD with MD-CTA, corresponding thin-cap fibroatheroma with superficial confluent necrotic core and calcium respectively red- and white-labelled by VH (bottom left).
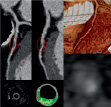
Figure 3. Orthogonal curved multiplanar reconstructions (top left), axial image (bottom right) and volume rendered image (top right) of a non-calcified plaque in the LAD with MD-CTA, corresponding fibroatheroma with mainly fibrous and fibro-fatty tissue composition respectively green- and greenish yellow-labelled by VH (bottom left).
Kappa value for inter-observer variability was 0.61 (p<0.0001).
Plaque type varied according to the clinical presentation with a higher incidence of mixed plaques among UA/NSTEMI lesions (76% vs 37%, p=0.04), while non-calcified plaques were more frequent in stable angina (63% vs 24%, p=0.04).
Comparison between MD-CTA and VH IVUS
The length of the segments analysed by VH IVUS was 18±9 mm (range 5.6 to 46.3 mm) and total plaque burden was 50.7±9.6% (range 25.4 to 63.6%). The per lesion analysis showed a significant difference in plaque composition between mixed and non-calcified plaques by MD-CTA (Figure 4).
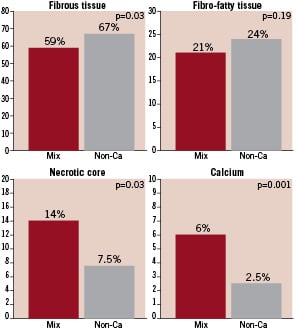
Figure 4. Plaque composition by VH-IVUS in mixed and non-calcified plaques.
Calcium and necrotic core, as a percentage of total plaque volume, were significantly higher in mixed plaques compared to non-calcified plaques (p=0.001), while fibrous tissue was significantly higher in non-calcified plaques (p= 0.03). There was no significant difference in fibro-fatty content.
No significant differences (p=0.28) were found in plaque burden between mixed and non-calcified lesions (50.0±12.2 vs 46.5±13.1%).
The three-frame analysis revealed a higher frequency of pathological intimal thickening in non-calcified plaques compared to mixed plaques (Figure 5).
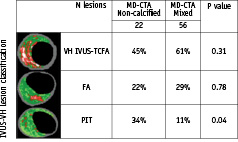
Figure 5. Distribution of plaque type by VH-IVUS lesion classification vs MD-CTA.
Focusing on the main characteristics of TCFA as identified by VH IVUS analysis (necrotic core >10%, dense calcium >5%, remodelling index >1.05), mixed plaques showed a higher incidence of lesions with calcium >5% (p=0.002), while other features were not significantly different between plaque types, as identifiable on MD-CTA (Table 2).
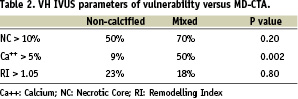
The positive predictive value of MD-CTA for the detection of vulnerable plaques (VH IVUS-TCFA) was 77%. This is because only 61% of all mixed plaques show vulnerability features, while VH IVUS-TCFA was present in 45% of non-calcified plaques. The negative predictive value was 54% with a global diagnostic accuracy of 59%. Of note, 23% of non-calcified plaques show positive remodelling.
Discussion
CAD is a chronic disease in which acute episodes associated with plaque events may cause sudden ischaemic cardiac death and myocardial infarction. Tissue composition of coronary plaque is known to play a decisive role in its propensity to cause acute events, the so-called “vulnerable plaque”.11,12,17-21 Necrotic core and calcifications are known to correlate with plaque progression and portend an increased risk of acute coronary events.19,20 Most knowledge about plaque features that cause acute coronary events is provided by retrospective histopathological studies of patients presenting with fatal episodes.11,12,17 For the first time, VH IVUS is providing an opportunity for in vivo analysis of plaque burden and composition during the lifetime. VH IVUS provides a morphological evaluation of atherosclerotic plaque by quantifying its different components in absolute and relative terms and by determining their cross-sectional location in relation to lumen. Presently, the epidemiology of vulnerable plaque is being investigated in various patient subsets and in vivo findings from several imaging tools are starting to be cross-correlated and compared to the results of biomarker studies.22 In addition, the natural history of various plaque types and their response to targeted drug therapy is currently being examined in prospective studies.23 However, acquiring VH IVUS data still requires an invasive evaluation, meaning that it will not be applicable as a screening method in subjects with pre-clinical disease, or even in patients with known CAD but in whom an invasive procedure is not justified on clinical grounds. With the recent emergence of “non-invasive” coronary angiography through the development of MD-CTA, CAD can be diagnosed at pre-clinical or early stages, with the potential to identify high-risk plaques and the promise that acute events could potentially be prevented by appropriate medical and behavioural management. Central to the validity of this hypothesis is the assumption that vulnerable plaque can indeed be identified by MD-CTA. The purpose of our study was therefore to characterise plaque type by MD-CTA and to correlate this classification with the VH IVUS determination of plaque composition, as the currently available best standard for in vivo identification of coronary lesions with high propensity for causing acute events. In an attempt to standardise lesion classification by MD-CTA, objective criteria based on density measurements (HU) were applied resulting in good inter-observer variability (Kappa value >0.6). The ability to discriminate between non-calcified and mixed plaques with MD-CTA is confirmed by the highly significant difference in calcium content as measured by VH IVUS.
The main findings of this study are follows:
Plaque characterisation presently available from MD-CTA correlates fairly well to the more detailed tissue analysis provided by VH IVUS:
a. indices of increased vulnerability, such as higher content of necrotic core and calcium are seen significantly more often in mixed, than in non-calcified plaques by MD-CTA.
b. Mixed plaques by MD-CTA are seen significantly more often in patients presenting with acute forms of CAD.
However, simple classification of plaques by MD-CTA as calcified, non-calcified or mixed did not provide the high diagnostic accuracy that would be required to support its use for the non-invasive detection of vulnerable coronary plaque. Importantly, 23% of non-calcified plaques show positive remodelling, which is also associated with increased risk of events.24
Comparison with previous studies
No comparisons between MD-CTA and VH IVUS have been reported so far. Previous studies have used grey scale IVUS and have shown that plaques with low echogenicity (presumably lipid rich) are mostly soft (non calcified) by MD-CTA.4-6 Our study, which uses VH IVUS as a more detailed standard for plaque characterisation, shows in contradiction, that mixed plaques (instead of non calcified) are more likely to show vulnerability features.
Study limitations
The characterisation of plaque with MD-CTA suffers from a number of shortcomings.
First, assigning a plaque type to a specific range of densities is fraught with some problems. The attenuation of soft plaque is known to change with the attenuation of the contrast-enhanced lumen, which contributes to increasing the existing overlap between mean density values.16,25,26
Second, spatial resolution of MD-CTA is relatively low resulting in partial volume artefact.
Third, recently described dedicated software for automatic measurement of plaque density and volume were not available to us and could be helpful.27
Fourth, the presence of thrombus can affect the border detection analysis. When present, fresh thrombus needs to be excluded at the time of contour detection. In this study, only 29% of included patients presenting with acute coronary syndrome had increased troponin levels. Invasive studies were not performed in the acute phase (upon hospital admission) nor without prior systemic antithrombotic and antiplatelet therapy. Actually no evidence of fresh thrombus was detected in the present study.
Fifth, we found only a trend suggesting that MD-CTA can identify vulnerability features of individual stenoses. Given the inclusion of a limited number of patients, the possibility of a type II error should be considered. However, the analysis is based on a large number of separate plaques (n=78). Increasing the patient population will likely improve the statistics on the positive findings but will not erase the intrinsic shortcomings of plaque characterisation by current MD-CTA technology, i.e. the large proportion of vulnerability features that were seen in non-calcified plaques.
Lastly, the present analysis was not intended to provide outcome data but was rather designed as a pilot study to validate and explore the accuracy of MD-CTA in order to help design and calculate sample sizes that will be required for prospective studies. From the present study, it already appears that the discriminate power of MD-CTA is not such that this technology could be proposed as the sole tool for patient selection in outcome-based trials targeting modification of the vulnerable plaque.

