Abstract
Aims: The purpose of this study was to evaluate the feasibility and short-term efficacy of transcatheter paravalvular leak closure using different occlusion devices.
Methods and results: Twenty-one patients underwent transcatheter closure of either aortic or mitral paravalvular leak from June 2002 to February 2006 using the Amplatzer PDA, ASD or VSD occluder. All patients had symptoms and signs of haemolysis and/or cardiac decompensation with dyspnoea.
Implantation of a device was technically successful in twenty patients (95%). Immediate residual leak was found in seventeen patients (85%). Significant shunting persisted in nine cases during follow up (45%).
Permanent leaflet obstruction was observed in one patient. Severe complications during follow up led to early death in one patient and surgical intervention in three. A successful second catheter treatment was performed in another three patients. The event-free survival from re-operation, death and stroke at the end of the observation period was 80%.
Conclusion: Transcatheter closure of paravalvular leaks is a technically feasible, but demanding procedure. Residual leaks are common and may worsen pre-existing haemolysis. Due to the significant ongoing morbidity in this group of patients and the complexity of follow up individual patient results differ considerably. Nevertheless, it is possible to achieve some symptomatic relief, thus an interventional approach should be discussed as a potential treatment option for those patients with a limited defect and who are not deemed suitable for another operation.
Introduction
Congenital or acquired valve disease may result in severe and symptomatic cardiac dysfunction such that surgical repair or replacement of one or more of the valves is unavoidable. Results after surgical valve replacement are generally good with symptomatic relief and acceptable morbidity, but complications do occur. One such complication is persistence or development of a paravalvular leak. Localised around the circumference of the valve sewing ring, these defects cause regurgitation and may lead to cardiac decompensation. Patients with paravalvular leaks are also at risk of sustaining mechanical haemolysis causing severe anaemia and requiring repeated blood transfusion. Surgical closure of these leaks is feasible and remains the gold standard, but with the advent of newer interventional occlusion devices, designed for closure of the patent arterial duct, atrial septal defects and ventricular septal defects, it is now possible to close some of these leaks at cardiac catheterisation.
Population
Twenty-one patients aged 46 to 83 (8 females and 13 males, mean age 65.5±8.8 years) received transcatheter treatment of paravalvular leakage from June 2002 to February 2006 at our institute (Table 1).
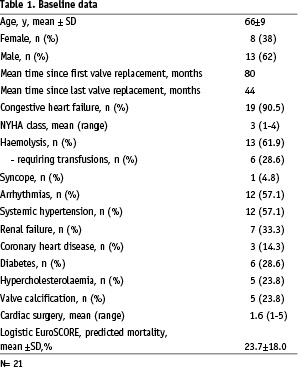
The majority of patients were deemed unsuitable for re-operation due to a high predicted mortality rate. Some patients were offered surgery, but declined. Thirty eight percent (8/21) of the patient group had had one or more surgical redo procedures before catheter treatment was considered. As an example of a protracted clinical course, one patient developed obstructive thrombi on two mechanical valves nine years after valve replacement surgery. Several months later, re-operation was required due to endocarditis. Another patient underwent five operations in a four year time span because of severe paravalvular leakage.
The overall logistic EuroSCORE1 predicted mortality was around 24%. Considering that redo surgery or double/triple valve replacement is not recorded by the EuroSCORE calculation, the actual predicted mortality may even exceed this value. Mitral paravalvular leakage was present in thirteen patients (62%) and aortic leakage in eight (38%). Four patients with mitral and one patient with aortic valve replacement had multiple leaks around the mechanical prosthesis. Multiple leaks (n=5; 23.8%) were always associated with a substantial amount of haemolysis, necessitating blood transfusions in all five patients.
Fluoroscopic leak diameter ranged from 2-10 mm (n=18; mean 5.1±2.3 mm). Three patients with a very small leak did not receive standardised fluoroscopy measurement. Defects around bioprostheses (n=4) were in 3 of 4 patients >8 mm. The largest leak around a mechanical valve measured 6mm. The following forms of paravalvular leakage could be differentiated:
– Multiple perforated valve circumference
– Crescentic defects
– Single small leak with a high velocity jet
Unusual pathology:
– Patch dehiscence causing mitral paravalvular regurgitation
– Para-aortic regurgitation through a connection between left ventricular outflow tract and left atrium
The haemodynamic effects of regurgitation varied with the type of defect. All patients with mitral paravalvular leakage had left atrial dilation > 45 mm and pulmonary hypertension (mean pulmonary artery pressure > 25 mmHg at rest). Apart from one patient, all patients with an aortic paravalvular leak had a dilated left ventricle > 55 mm. The exception was a patient in whom transoesophageal echo confirmed a paravalvular leak with flow from the left ventricular outflow tract to a dilated left atrium, depicted in figures 1 and 2.
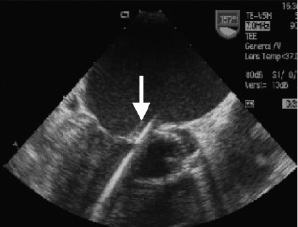
Figure 1. Probing the defect. Transoesophageal echocardiogram in 155° angle. Medtronic Hall valve in aortic position. Catheter (arrow) crossed the paravalvular leak and is stabilised in the left ventricular apex.
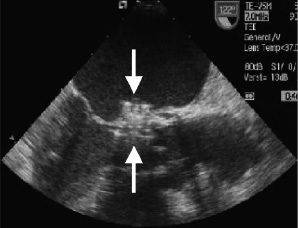
Figure 2. Device deployed Transoesophageal echocardiogram in 120° angle. An Amplatzer-VSD device is deployed in the leak (arrows).
Another patient subjectively felt no permanent symptoms, but had a dilated left ventricle (58 mm) and borderline myocardial function (EF: 53%), as well as elevated lactate dehydrogenase levels (304U/L) and recurrent syncope.
All patients gave informed consent to a transcatheter paravalvular leak approach.
Methods and techniques
Patients underwent standard imaging with transthoracic echocardiography and transoesophageal echo and occasionally cardiac magnetic resonance imaging or intracardiac echocardiography. Paravalvular regurgitation was quantified by colour Doppler to assess regurgitant fraction and/or regurgitant volume in all patients. Haemolysis was diagnosed mainly on haemoglobin levels (<100 g/l lasting at least two weeks without other cause). Pre-interventional medication included endocarditis prophylaxis (e.g. cefuroxime 1.5 g, i.v.) and 5,000 to 10,000 units of heparin. Almost all procedures were performed under local anaesthesia, unless general anaesthesia was especially requested by the patient. Atropine and propofol were administered before intraoperative transoesophageal echo. Angiography was performed during the catheter procedure to elucidate the position of the leak. Six to eight size French Cook shuttle sheaths (Cook Inc., Bloomington, IN, USA) were found to be particularly helpful for device delivery.
For closure of aortic paravalvular leakage, retrograde access via the femoral artery was standard. Aortography was performed with a pigtail catheter. An appropriate end hole catheter such as a multi-purpose, right Judkins coronary catheter with a Terumo wire (Terumo Medical Corp., Somerset, NJ, USA) was used to cross the leak to the left ventricle, using fluoroscopy and transoesophageal echo to avoid crossing the prosthetic valve. Balloon sizing of the defect was performed with either gentle inflation of coronary angioplasty balloons or one of the smaller size coaxial angioplasty balloons such as the Tyshak series (NuMed, Cornwall, Ontario, Canada). Fluoroscopy measurement of the balloon indentation pointed out the silhouette and diameter of the defect and thereby governed device selection. Using an exchange length wire, the relevant delivery sheath for the selected device was positioned in the left ventricle. The device was detached in the usual fashion by extruding the distal disc of the device in the cavity of the left ventricle, pulling the device to the ventricular aspect of the valve ring, and then delivering the proximal disc of the device on the aortic side of the valve ring. Proximity of the paravalvular defect to the coronary ostia required efficient aortography to assure continuous coronary flow. Once appropriate device position was achieved, the device was released. For small aortic leaks the Amplatzer PDA occluder (AGA Medical Corporation, Golden Valley, USA) was selected and for medium to large defects the double disc Amplatzer muscular VSD device provided effectiveness and stability.
Transcatheter mitral paravalvular leak closure was mostly achieved via the inferior vena cava, employing a transseptal puncture approach to left atrium. In the case of a paravalvular leak close to the atrial septum, a transseptal approach through the superior vena cava or a retrograde approach from the femoral artery, aorta and left ventricle was required. Retrograde access was sometimes complicated by left ventricular structures of trabeculae, papillary muscles, and chordae. A complete arteriovenous circuit was necessary in three patients to maintain stability and allow device advancement from either the arterial or the venous side.
Typically, the lesion was crossed from left atrium to left ventricle with an end hole diagnostic catheter and a hydrophilic Terumo wire to minimise friction. Probing the leak on occasions was technically difficult and time consuming. The Terumo wire was substituted for an exchange length guidewire allowing a delivery sheath to be advanced. The appropriate sized device was then deployed with the distal disc on the left ventricular aspect of the mitral valve and the proximal disc on the left atrial side (Video 1). During the implantation sequence, the position of the device was constantly guided by transoesophageal echo. Flow profiles around the occluder and residual shunting were scrutinised by colour flow Doppler before final detachment. The most useful angiographic projections for implantation were between 15-20° RAO and 20-40° caudal for both mitral and aortic procedures.
Follow-up was scheduled at 4 weeks and 6 months. Depending on the patients’ cardiac status and alteration of symptoms, the follow up examinations were adjusted individually. Cardiovascular supervision after intervention included transoesophageal echo, electrocardiography, transthoracic echocardiography, chest x-ray in two projections and regular assessment of haemolysis, infection and retention parameters. Extended follow-up was performed by questionnaires or personal telephone calls.
Residual shunting was regarded as ‘significant’ when clinical symptoms of haemolysis and/or cardiac decompensation occurred in combination with high velocity paravalvular jets or paravalvular regurgitation >2nd degree (regurgitant fraction >20% or regurgitation volume >30 ml). Haemolysis was classified as ‘Significant’ when haemolysis blood parameters (haemoglobin, lactate dehydrogenase, haptoglobin, indirect bilirubin) deteriorated during the post interventional course and/or transfusions were utilised at any time during follow up and no other cause for anaemia could be identified.
Results
On average, transcatheter paravalvular leak closure was performed 44±37 months after the last surgical valve replacement (Table 2).
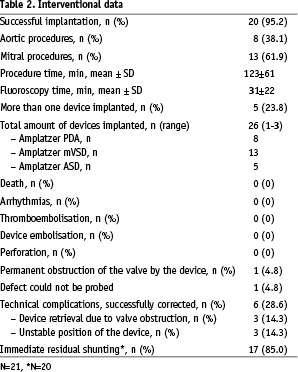
The procedure was technically successful in twenty of twenty-one patients. In three patients a second transcatheter procedure to close remaining leaks was required and was successfully performed 6 months after the first implantation. Summarising all interventions, four patients received two occluding devices (Figure 3) and one patient three (Figure 4), resulting in 26 devices in 20 patients.
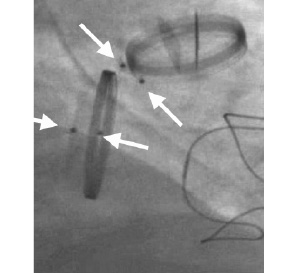
Figure 3. Multiple leak closure in mitral position. Fluoroscopy view obtained from a patient with two mechanical valves and one tricuspid xenograft. Occluding devices are positioned around the mitral circumference (arrows).
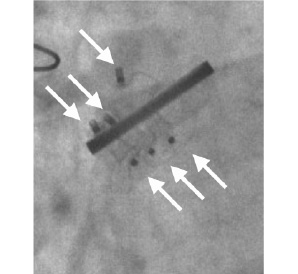
Figure 4. Multiple leak closure in aortic position. Fluoroscopy view of three Amplatzer VSD devices positioned around a mechanical St. Jude Medical aortic valve.
The device sizes ranged from 4-14 mm. Mean procedure time was 122.6±61.4 min and mean fluoroscopy time 30.5±21.9 min.
Device implantation failed in one patient with a paravalvular mitral leak. This patient presented difficult access problems due to a tough, thickened atrial septum necessitating dilation with coronary angioplasty catheters prior to access with a diagnostic catheter. Despite prolonged efforts with various diagnostic catheters and wires, it was not possible to cross the leak. This patient was later referred for cardiac transplantation.
During two mitral procedures, the device interfered with a mechanical valve leaflet. The devices were retrieved before complete delivery and replaced with smaller occluders. Three other devices were replaced due to an unstable position or an incomplete or distorted constitution profile (Figure 5).
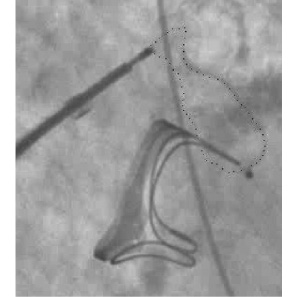
Figure 5. Distorted Amplatzer device. Fluoroscopy view of a distorted Amplatzer VSD device (silhouette marked) located aside a Baxter Perimount mitral bioprosthesis.
In one patient a major intraprocedural complication occurred. Though the correct position of the device had been assured before release (Figure 6a), the device changed its configuration after disconnection. Figure 6b depicts the effect of this displacement with consecutive obstruction of the inferior valve leaflet (Video 2).
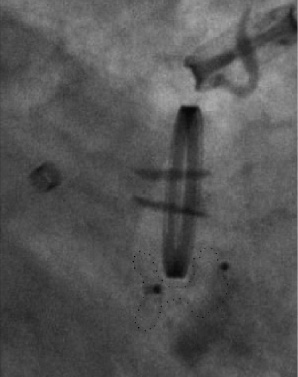
Figure 6a. No leaflet interference. Fluoroscopy view of an Amplatzer VSD occluder (silhouette marked) shortly after releasing the device. No leaflet obstruction.
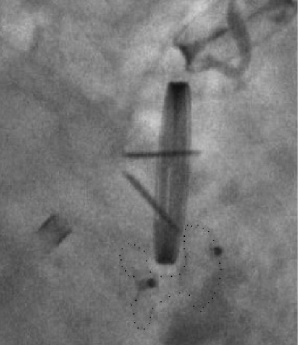
Figure 6b. Permanent leaflet interference. Fluoroscopy view of an Amplatzer VSD occluder (silhouette marked) obstructing the inferior leaflet of a St. Jude Medical mechanical valve. The configuration of the device changed after releasing the device.
New haemolysis occurred shortly after the procedure requiring transfusions every 10 days. Four months after intervention this patient underwent surgery, but died 22 hours after the procedure.
There were no other complications during the interventions. Specifically there was no heart block, no thromboembolism, and no device embolisation or bleeding complications, all of which have been reported previously2,3. Seventeen of twenty patients (85%) had immediate residual leakage (Video 3).
Table 3 displays the follow up data.
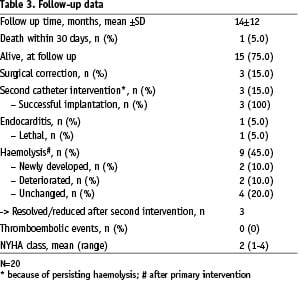
Mean follow up time (n=20, patient 21 excluded) was 13.5±12.2 months with a total of 22.5 patient years. Five patients developed severe complications after the procedure:
Prosthesis endocarditis accompanied by acute subarachnoid haemorrhage led to the death of a patient 20 days after the procedure.
After interdisciplinary consultation, two patients decided to undergo a surgical re-operation because a severe haemolysis persisted or worsened post procedurally. In another case the early interventional result was promising; however, a dehiscence with distinct paravalvular regurgitation was found by transoesophageal echo at 7.5 months. As mentioned earlier, one patient died from the consequences of re-operation after leaflet obstruction by the device.
Three patients with ongoing haemolysis were treated with a second catheter device implantation. The reintervention was technically successful in all patients and resulted in a cessation (n=2) or relevant reduction (n=1) of haemolysis.
In four patients, haemolysis worsened or newly developed after the procedure. This type of haemolysis seemed to be attributed to a high flow rate through the occluder wire mesh, thereby causing mechanical fragmentation of the erythrocytes. When post-procedural haemolysis occurred, treatment options were discussed with the patient. Slight, non progressive haemolysis was observed until endothelialisation of the device framework, which is usually complete at 6 months. Patients with more haemolysis or rapidly deteriorating blood parameters were assessed and assigned to a second intervention or surgery.
Interventional success in terms of declining symptoms (objectified by NYHA class, echocardiography and haemolysis parameters) was found in fourteen patients (70%), including three patients that received a second procedure. Another two patients improved in cardiac function, but continued having haemolysis. Both have a second intervention planned. Defining re-operation, death and stroke as major adverse clinical events, the event-free survival at the end of the observation period was 80%. By including also significant residual shunting and reintervention, the event free survival decreases to 50%.
Despite successful paravalvular leak occlusion and a decrease in symptoms, three patients died from heart failure during the course of follow-up.
Review and discussion
There are several reasons for early tissue dehiscence around an implanted valve leading to a paravalvular leak. The retention sutures may loosen, rupture or tear apart from the surrounding tissue and this suture-tissue integrity can be compromised by calcification, infective endocarditis or inherent tissue friability4,5.
Infective endocarditis is regarded as the most significant risk factor for paravalvular leakage6-8. Postoperative infective endocarditis correlates with increased re-operation mortality and with the appearance of larger and multiple paravalvular leaks9,10. The probability of valve infection is similar between bioprostheses and mechanical valves11.
In about 60% of cases paravalvular leakage occurs within the first year after surgery. In a review of five publications on valve replacement with a minimum of 1,000 patients4,10,12-14, the total frequency of paraprosthetic leakage ranges between 0.75 and 2.3% (mean 1.7%±0.7%).
One of the largest collections of surgical paravalvular leak patients was presented by Genoni et al in 19919. Out of 95 mitral paravalvular leak patients, 50 received surgery. The overall surgical mortality rate was 12%, considerably less than 26% for patients treated medically.
Early data on transcatheter valve replacement with the Cribier-Edwards valve reported an incidence of 25% for medium and large paravalvular leaks. Currently larger prototypes of transcatheter valves are in investigational use. Paravalvular leak incidence seems to be significantly lower with this new generation of valves15.
Table 4 lists the current literature on transcatheter paravalvular leak closure.
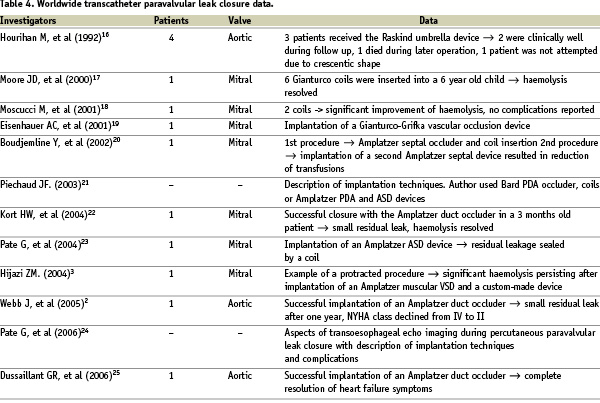
Hourihan was the first author to describe transcatheter experience of closing leaks in a group of four patients in 199216. In three patients, the Rashkind double umbrella device was successfully implanted, whilst in one case the occluder could not be positioned due to the crescentic shape of the defect. Two of four patients were clinically well during follow up and the other two patients died during non-device associated surgery. Moore used 6 Gianturco coils to occlude a leak in a 6 year old child in 2000, leading to a relief of haemolysis17. In 2001 Moscucci reported successful occlusion of a mitral paravalvular leak in a 55 year old man with two artificial valves, endocarditis and annular calcification18. After transseptal puncture two dumbbell coils were inserted into the leak, relieving the patient of severe haemolysis. Since then, there have been several other case reports on paravalvular leak closure presenting novel approaches with different devices such as the CardioSEAL Clamshell device (Nitinol Medical Technologies, Boston, MA, USA), the Gianturco-Grifka vascular occlusion device (Cook Cardiology, Bloomington, IN, USA), the Amplatzer PDA, VSD, ASD or vascular plug (Amplatzer Medical Corp., Golden Valley, MN, USA)2,3,19-25. The difficulties encountered during percutaneous closure of paravalvular leakage have been described3. Complications of device embolisation, thromboembolism, infection, haemolysis and interference with adjacent valve leaflets may spoil an apparent technically sound procedure24.
Comment
The indication for a transcatheter approach in our patients consisted of a critical analysis of the patient’s symptoms, the clinical course and physical status. Patients with an unstable valve or active endocarditis were not considered for a catheter intervention. Experience has shown that best results may be achieved in patients with a small to medium sized (<5 mm), single, circular paravalvular leak with a distance of at least some millimetres from the valve margin.
Paravalvular leak appeared in various forms, depending on the individual aetiology. Crescentic defects were most frequently encountered and often located directly at the valve rim. As a consequence, interference with leaflet function was more likely with this type of defect. Since the Amplatzer occluder series is not capable of entirely sealing large crescentic defects without leaflet obstruction, residual shunting and haemolysis occurred more frequently in this subgroup. Consecutively, a second device was needed to achieve competent occlusion. Transoesophageal, transthoracic and also intracardiac echo may provide limited anatomical resolution of paravalvular leaks. Three-dimensional echocardiography may be of some help in addressing this difficult imaging issue.
Though the Amplatzer series has potential advantages of filling defects of different morphology and diameter with its flexible nitinol waist, our results would suggest that defect specific occluding systems with a crescentic configuration and impermeable waist are required to produce better acute and longer term results for this pathology.
Conclusion
Interventional paravalvular leak occlusion, by necessity, has been performed only relatively recently and in a highly selected patient group. Ongoing clinical and investigative scrutiny is required to assure safety and efficacy. The complexity of these defects presents a technical challenge and the procedures are often long and complicated. An effective result may require multiple device implantations as a single or staged procedure. Even so, persisting or even increasing haemolysis, endocarditis or a need for surgery was encountered during follow up. Despite the potential limitations of interventional closure of paravalvular leaks with existing devices, most patients experience an improvement in symptoms. Thus this technique should be considered a treatment option for patients with paravalvular leaks with limited defects who carry an unacceptable surgical risk.
Online data supplements
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.
Moving image 2.
Moving image 3.

