Abstract
Aims: To evaluate the feasibility of lesion related treatment strategies in patients treated for severe carotid artery disease with angioplasty and stenting (CAS) under embolic protection devices (EPD).
Methods and results: From December 2001 to August 2004 a total of 377 consecutive patients were enrolled to undergo CAS. The procedure was conducted by using several types of stents (cobalt-alloy frame, nitinol frame) and of EPD (filter-wires, proximal endovascular clamping devices) applied to specific lesions and/or anatomies.
Primary endpoint was to assess the death and stroke rate at discharge.
Secondary endpoints were to test the feasibility and safety of tailored CAS (angiographic success, any complication between discharge and 30 days, death of any cause at 30 days).
The procedural success was achieved in 377/377 patients (100%).
Adverse events included:
1. during procedure: 2 TIAs (0.53%)
2. at discharge: 1 death procedure related (0.27%), 1 major stroke (0.27%), 2 minor strokes (0.53%), 4 TIAs (1.06%), 1 intracranial hemorrhage (0.27%); all adverse event rate at discharge 2.92%; all strokes and death rate at discharge 1.06%;
3. at 30 day f.u.: one death not procedure related (0.27%), 1 minor stroke (0.27%);
4. overall procedure related stroke and death rate: 1.33%.
Conclusions: Our data suggest that using new materials/devices matched to specific lesions or anatomies is safe and effective.
Introduction
Carotid angioplasty and stenting (CAS) is becoming more widely performed for the treatment of severe carotid obstructive disease, and is now accepted as a less invasive technique that provides an attractive alternative for many patients, particularly those with significant co-morbidities1-8.
Nevertheless, no data are actually available about the correct use of specific devices. Each stent and protection device has its own technical features. We believe that the different devices should be used following pre-defined logic indications rather than be chosen by chance.
In presence of different types of stents and embolic protection devices (EPDs), the CAS strategy could consist in a process of tailoring the endovascular procedure to a specific patient and a specific kind of carotid lesion and vascular anatomy (“tailored” CAS strategy).
This study was designed to evaluate the feasibility of new materials and devices, and associated treatment strategies in patients percutaneously treated for carotid artery disease using distal protection.
Methods
From December 2001 to August 2004 a total of 377 consecutive patients, were enrolled to undergo protected stenting of the extra-cranial carotid artery based on an individual treatment strategy.
A written informed consent for intervention was obtained from all patients.
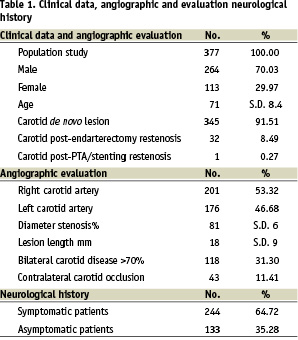
The demographic, clinical data, angiographic evaluation and neurological history of the study group are summarized in Table 1.
Patient inclusion criteria:
1. Patients age > 50 years old.
2. Patients with symptomatic > 70% carotid stenosis
3. Patients with asymptomatic > 80% carotid stenosis
4. Bilateral carotid critical stenosis > 70%, symptomatic or asymptomatic
5. Patients refused by surgeons as high-risk surgical subsets
Patients exclusion criteria:
1. Patients with thrombocytopenia, leucopenia, neutropenia or gastrointestinal bleeding in the previous three months
2. Patients with an allergy to aspirin or clopidogrel or ticlopidine
3. Angiographic appearance of fresh thrombus at the carotid lesion site
4. Angiographic appearance of carotid chronic total occlusion
Patient assessment
All neurological examinations were performed by an independent neurologist team.
Prior to treatment, all patients underwent careful neurological examination, echo/color flow Doppler, cerebral CT/MR scan.
Within 24 hours following the procedure and at 30 day post-discharge all the patients underwent a neurological examination and a complete echo/color flow Doppler evaluation.
A post-procedure cerebral CT/MR scan was performed only in patients with documented neurological complications.
Pre-procedure echo-analysis of carotid plaques (lesions characteristics)
In all these patients echolucency was measured by using the gray scale median (GSM), a computer-assisted grading of the echogenicity of carotid plaques15-17. When GSM value was below 25, the carotid lesions were categorized as soft plaques at high risk for peri-procedural embolic complications18.
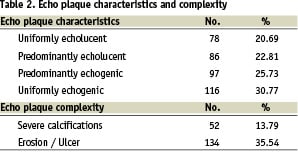
The echo-plaque characteristics and complexity are summed up in Table 2.
Scheme for device selection
The use of specific devices applied to specific lesions and/or anatomies satisfied the general concept of matching the technical features of each material/device to the carotid lesion characteristics (“tailored” CAS).
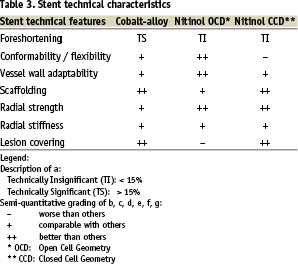
The stent technical features are different and vary thoroughly if we compare cobalt-alloy braided thread frames, nitinol open cell design and nitinol closed cell design frames.
In the present series, we took into account the following stents:
1. cobalt-alloy braided thread structure: Carotid Wallstent (Boston Scientific)
2. nitinol open cell design: Acculink (Guidant), Exponent (Medtronic), Protegè (ev3), Conformex (Bard), Smart Precise (Cordis)
3. nitinol closed cell design: Xact (Abbott)
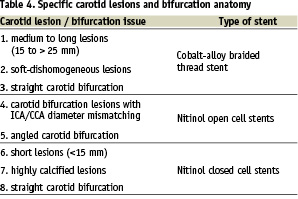
Based on the described stent technical characteristics in Table 3, the operators assigned the stenting strategies to the specific carotid lesions and bifurcation anatomy in Table 4.
In order to assign the specific embolic protection device (EPD) to the specific carotid lesion/vascular anatomy, the operators followed neuroprotection strategies as shown in Table 5.
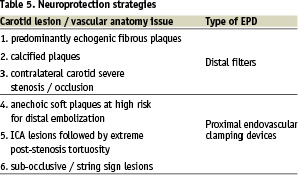
Procedure strategy remark: in the present series no distal balloon occlusive devices were used.
Definitions
1. Procedural success of protected carotid stent deployment was defined as:
• angiography: less than 30% residual diameter stenosis of all treated lesions, without alterations in the intracranial circulation at the post-procedural angiographic examination (the assessment of the residual diameter stenosis was performed by averaging at least two matched views on quantitative angiography);
• echo color-Doppler: absence of significant residual stenosis and pathological acceleration in blood flow (less than 1.5 m/sec).
2. A transient ischemic attack (TIA) was defined as a focal retinal or hemispheric event from which the patient made a complete recovery within 24 hours.
3. A minor stroke was defined as a new neurological deficit that either resolved completely within 30 days or increased the NIH Stroke Scale by < 3.4.
4. A major stroke was defined as a new neurological deficit that persist > 30 days and increased the NIH Stroke Scale by > 4.
5. A fatal stroke was defined as death attributed to an ischemic stroke or intra-cerebral hemorrhagic stroke.
Medical treatment
Pre procedure:
All patients were treated with acetyl-salicylic acid (ASA) at a mean dosage of 125 mg/die associated with clopidogrel or ticlopidine at a mean dosage respectively of 75 mg/die or of 500 mg/die at least 4-5 days prior to admission.
During the procedure:
The mean dosage of sodium heparin utilized during the procedure was 70 U per kg.
Just before the post-stenting dilation phase, atropine (0.5-1 mg IV) was given to the most patients in order to reduce the bradycardia and hypotension potentially associated with carotid dilation. Atropine wasn’t administered in patients with tachycardia and uncontrolled systemic hypertension.
Post procedure:
Clopidogrel (75 mg/die) or Ticlopidine (500 mg/die) was continued for at least 30 days after the interventional procedure (hemochrome and white blood count were checked 7-10 days following the percutaneous intervention).
Mono anti-platelet therapy (either aspirin or clopidogrel or ticlopidine) is being continued indefinitely.
Description of the procedure
All the protected procedures were carried out via puncture of the right and/or left femoral artery.
Based on our experience with percutaneous coronary angioplasty and stenting, the common carotid artery was selectively engaged directly by using a proper 8 F guiding catheter.
When the use of a primary guide catheter was not possible, due to the particular anatomy of supra-aortic vessels, we placed a stiff wire into the external carotid artery, for positioning of a long sheath or a guiding catheter (co-axial technique) into the common carotid artery.
All patients underwent an angiographic examination of the culprit carotid lesion in two different projections and an angiographic examination of intra-cranial circulation in antero-posterior and/or lateral projection. The same angiographic check-up was performed at the end of the procedure in order to determine if there was any variation in the intra-cranial blood flow.
Carotid stenting
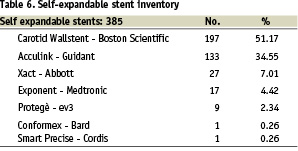
Carotid stenting was carried out by using self-expandable stents in all 377 cases.
Stent details are located in Table 6.
In tight, sub-occlusive carotid stenoses pre-dilation was performed with low profile balloons 0.014 wire compatible.
The pre-dilation balloons were routinely undersized (artery/balloon ratio: 1.8-1.5) in order to reduce vessel dissection and/or distal embolization.
Specifically for heavily calcified lesions, predilatation was performed by using a coronary 4.0 mm Cutting Balloon (Boston Scientific).
Stent placement was optimized through single or multiple dilations by using suitably sized balloons based on angiographic quantitative analysis of the vessel.
Cerebral protection devices
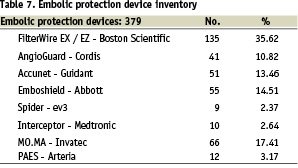
In the present series many cerebral protection devices (filter-wire devices, reversal flow and proximal occlusive devices) were used.
For details regarding cerebral protection devices see Table 7.
Results
Procedural results are reported in Table 8.
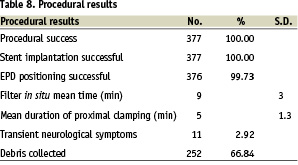
Procedural success was achieved in 377/377 subjects (100%).
Stent implantation was successful in all cases. For obtaining an excellent final result, in 8 cases (2.1%) an adjunctive stent was implanted.
Embolic protection device positioning was successful in all cases but one (99.73%). In this case an AngioGuard (Cordis) 6 mm was planned to be delivered beyond a severe 85% focal heavily calcified angled lesion. Despite numerous attempts to cross the lesion, the operator wasn’t able to cross the calcified plaque with the stiff body of the constrained nitinol AngioGuard cage. For this reason the planned device was exchanged with an EPI-filter EZ (Boston Scientific), and this device allowed the operator to succeed.
In one case protected by using a MO.MA proximal protection system, the angiographic appearance of significant plaque prolapse through the implanted Acculink stent meshes after balloon post-dilatation required the positioning of a distal filter-wire (AngioGuard – Cordis 7 mm) and of an additional Carotid Wallstent 9/30 mm (Boston Scientific).
In-hospital adverse events
The complications are described in Table 9.
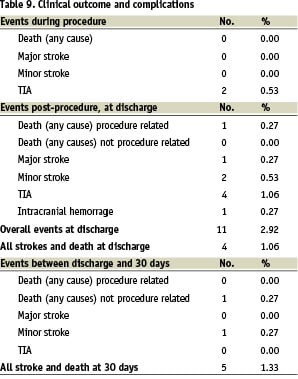
The reported neurological adverse events included:
1. during procedure: 2 TIAs (0.53%) both occurred immediately after proximal protection device (MO.MA) removal.
2. post-procedure at discharge: 1 death procedure related (0.27%) at day 6 post-procedure (bowel infarction related to diffuse cardio-embolization in a 80 year madam with chronic atrial fibrillation); 1 major stroke (0.27%), 2 minor strokes (0.53%), 4 TIAs (1.06%), 1 intracranial hemorrhage (0.27%).
All adverse event rate at discharge was 2.92%; all stroke and death rate at discharge was 1.06%.
No cerebral protection device related complications occurred in the present series.
The cases complicated by intra-cranial hemorrhage achieved complete resolution of symptoms (hemi-sensory loss, hemiplegia) and radiological findings (CT and MR) within 4 days.
Adverse events between discharge and 30 days
The complications are described in Table 9.
The reported clinical and neurological adverse events included:
1. one death not procedure related 0.27% at day 25 post-procedure (acute pulmonary edema in dilative cardiomyopathy);
2. 1 minor stroke (0.27%).
The overall procedure related death and any stroke rate at 30 days was 1.33%.
Overall adverse embolic events categorized for materials (embolic protection devices and stents)
The enrolled patients experienced a total of 10 embolic complications (2.65%). These complications were categorized for materials used for protected carotid stenting as shown in Table 10.
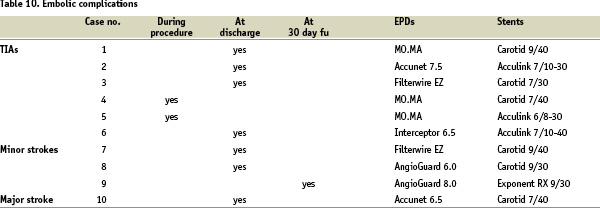
From a technical stand point, we can state that no significant correlation was observed between materials and occurred embolic complications: actually, we had embolic neurological adverse events with all the most used types of stents (cobalt alloy braided thread frame, nitinol open cell design frames, both cylindrical and tapered) as well as of embolic protection systems (filter-wires, proximal occlusion devices).
An important remark comes from the carotid plaque composition profile in this neurologically complicated subset: at echo-Doppler evaluation, all ten patients presented a pattern of soft plaque: uniformly echolucent in 3 subjects, and predominantly echolucent in the other seven subjects.
Complications and/or side effects related to the use of embolic protection devices
No major complications EPD related were reported in the present series.
Transient spasm of the intra-cranial segment of the carotid artery, presumably related to the protection device (filter-wire) was observed in 33 cases (8.75%). In all cases spasm was resolved after intra-carotid nitroglycerin administration (200-400 micrograms) and/or after EPD removal.
Neurological transient intolerance (loss of consciousness, tremors and fasciculations) were present in overall 11 subjects (2.92%).
Specifically regarding the subset protected by proximal protection systems (PAES, MO.MA), transient intolerance appeared in 3/78 cases (3.85%). In this subgroup the neurological symptoms manifested after a mean cerebral occlusion time of 5±1.3 minutes. Immediately after the occlusive system deflation, all the patients returned to baseline neurological conditions.
Carotid plaque debris removed by EPDs
In 252/377 cases (66.84%) macroscopically visible plaque debris was captured and retrieved.
Discussion
Numerous recent data in the literature have reported extensive case studies on patients treated with carotid angioplasty and stenting for critical carotid stenoses1-4,7-10.
Some recent papers5,7-9 have demonstrated that percutaneous treatment of carotid pathologies is correlated with a risk of cerebral ischemic events and, more generally, with a rate of complications that is no higher than those observed with traditional surgery.
In the last three years numerous stents and cerebral protection systems have been proposed in order to widen indications and to limit the acute neurological complications related to distal embolization.
Nevertheless, no data are actually available about the potential impact of technical features of stents and neuro-protection devices on CAS clinical outcome.
As mentioned before, the “tailored” CAS strategy bases its rationale mostly on deep knowledge of Patient clinical status, vascular anatomy, carotid plaque characteristics and complexity. We believe that the pathological conditions have to be matched to the technical features of the materials at disposition of the operator.
The presented study was designed to evaluate the feasibility of matching new materials and devices, and associated treatment strategies, to specific carotid lesions and anatomies.
Based on the scientific evidences emerging both from this study and literature data1-10, the following considerations should be taken into account:
1. All cerebral protection devices are developed to decrease the risk of distal embolization during CAS. At the moment, none of the commercially available devices can guarantee a complete emboli-free procedure.
2. As confirmed in other published experiences on protected CAS5-9, neither the proper stent nor the suitable EPD can effectively prevent late embolic complications (at 30 day follow-up: major stroke rate 0.27%, minor stroke rate 1.33%). For time distribution of adverse events see Graph 1.
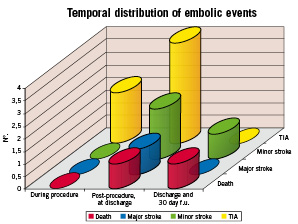
Graph 1. Temporal distribution of adverse embolic events.
3. In the awareness that neither the ideal stent nor the ideal neuro-protection device exist at the moment, the interventionalist, especially if at the beginning of his experience, should keep clear in his mind that carotid stenting has yet some unsolved technical limitations.
Study limitations
The patients did not undergo trans-cranial Doppler monitoring during the endovascular carotid procedure and did not receive a CT scan or MRI exam post-procedure For this reason, we are not able to provide any objective information about the following issues:
1. degree of embolization occurring while crossing the lesion with wires, balloons, stents and protection devices;
2. efficiency of the protection systems in capturing all the particles produced during carotid angioplasty and stenting.
Conclusions
Our data suggest that the use of new materials/devices matched to specific lesions or anatomies may improve both the procedural and 30 day clinical outcomes.
The new stents and embolic protection devices allowed operators to also treat more complex lesions and anatomies, thereby widening the indication for CAS.
“Tailored” percutaneous stenting of the carotid artery demonstrated to be feasible, but not without complications.
However, a quite long learning curve may exist for the proper use of different types of stents and embolic protection devices.

