Abstract
Aims: Cardiac Dimensions is developing a coronary sinus based percutaneous mitral annuloplasty device to treat functional mitral regurgitation called the CARILLON™ Mitral Contour System™.
Methods and results: A fixed length, double anchor device is advanced through a catheter to be positioned in the coronary sinus parallel to the posterior leaflet of the mitral valve. Once the distal anchor is deployed, tension is placed on the delivery system to plicate the peri-annular tissue and thus reduce the mitral valve diameter. Once mitral regurgitation reduction is optimized, a proximal anchor is deployed to maintain the annular reduction. Preclinical studies performed in tachycardia induced heart failure animal models have demonstrated both acute and chronic reduction of mitral regurgitation associated with device deployment. Haemodynamic and histological safety has been demonstrated over a 12 month period in healthy swine.
Conclusions: Percutaneous mitral valve annuloplasty is feasible. Initial safety and efficacy with the Cardiac Dimensions CARILLON™ Mitral Contour System™ has been demonstrated in preclinical studies. Clinical trials are required to elucidate the appropriate patient candidates for this novel therapy.
Introduction
Functional Mitral Regurgitation (FMR) is common in patients with advanced heart failure.1 FMR represents a dynamic pathology where alteration of the annular and subvalvular architecture contributes to mitral insufficiency, and that valve incompetence is frequently exacerbated by exercise. Lebrun et al. studied the changes of MR by proximal isovelocity surface area (PISA) and quantitative doppler studies in 27 patients with heart failure and functional MR who underwent exercise testing.2 They found that the regurgitant volume nearly doubled (from 21 ml to 39 ml) during exercise. A recent study by Peirard et al. documented exercise induced changes in effective regurgitant orifice area (EROA) and left ventricular ejection fraction (LVEF) associated with pulmonary oedema in patients undergoing exercise testing.3 Clearly, FMR represents a haemodynamic stress on the failing left ventricle that is intensified by exercise.
The impact of FMR on morbidity and mortality continues to be defined. Tada et al. evaluated 30 patients with dilated cardiomyopathy and NYHA class II or III to assess the effect of mild MR on exercise capacity in patients with congestive heart failure.4 They noted that the peak work load and oxygen uptake were significantly lower in the patients with MR, and they concluded that mild MR had a detrimental effect on the exercise capacity in patients with dilated cardiomyopathy. Blondheim et al. followed 91 patients with dilated cardiomyopathy longitudinally, and found that those patients with MR showed a markedly decreased survival (22% vs. 60% at 32 months), and the trend held true for mild MR as well.5 Specifically, the survival rates for patients with no MR was 59%, the survival rate for patients with mild MR was 26%, and the survival rate for patients with moderate or severe MR was 17%. These data show the correlation between MR, morbidity and mortality –and they influence the therapeutic recommendations advocated for these patients.
Amongst the surgical community, there is little controversy regarding the need to repair 3+ to 4+ MR if the patient is indicated for coronary artery bypass graft (CABG) surgery. A recent study by Lam et al. suggests that treating patients with 2+ (moderate) MR may be indicated as well.6 Their study assessed the impact of unrepaired moderate ischaemic MR. They found that moderate ischaemic MR does not resolve with CABG surgery alone and is associated with reduced survival; therefore, a mitral valve procedure may be warranted. Although performing mitral valve repair to reduce the symptomatology, volume overload and ventricular remodelling may be indicated in patients already indicated for open heart surgery, it is not currently recommended as a stand-alone procedure.7 Generating novel means to treat patients with functional mitral regurgitation via a minimally invasive approach has the potential to broaden the therapeutic options available to patients.
The favorable position of the coronary sinus and great cardiac vein (CS / GCV) parallel to the posterior leaflet of the mitral valve represents an attractive anatomy from which to develop a percutaneous therapy. Although the coronary sinus has a variable position superior to the mitral valve annulus,8 and although there exists an intimate relationship between the CS / GCV and the circumflex artery,9 early preclinical studies have demonstrated the feasibility of percutaneous mitral annuloplasty to treat FMR.10
Method
Cardiac Dimensions has developed a percutaneous mitral annuloplasty procedure which plicates the peri-annular tissue analogous to surgical mitral annuloplasty. The resultant reduction in annular dilation and septal-lateral dimension reduces the degree of mitral regurgitation.
Starting with internal jugular vein cannulation, the coronary sinus is cannulated with a 9F catheter (Figure 1a). Once a distal position in the CS/GCV is achieved, the nitinol device is advanced down the catheter to its target position. The device is comprised of a distal anchor and a proximal anchor. The distal anchor expands passively upon deployment, and is locked into its fully expanded position through use of the delivery catheter. The overexpansion of the anchor relative to the native venous dimensions facilitates its stability.
Once the distal anchor is deployed and locked, tension is placed on the delivery system (Figure 1b).
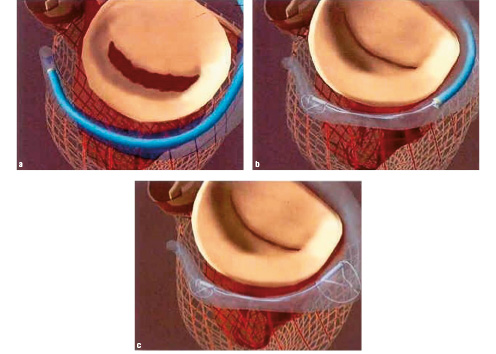
Figure 1. Procedure Overview: The CS / GCV is cannulated with a 9F delivery catheter. Once the distal anchor is deployed and locked, tension is placed on the system to plicate the peri-annular tissue and improve leaflet coaptation. Once the device position is optimized, the proximal anchor is deployed to secure the annular reduction.
This tension results in translation of the proximal anchor towards the CS ostium, and therefore creates tissue plication between the two anchors. Variable tension may be used to reduce the mitral valve dimensions by mild, moderate, or significant degrees depending upon the amount of annular reduction desired. Given the labile, load-dependent nature of MR, pharmacologic manipulation of the blood pressure may be warranted to confirm efficacy under various simulated physiologic conditions.
After confirming safety and efficacy, the proximal anchor is deployed in the coronary sinus, and locked into position in a manner analogous to the distal anchor (Figure 1c). Confirmation of safety and efficacy in this steady state guides the procedure. If haemodynamic assessments dictate the removal of the device, the delivery catheter can simply be advanced over both the proximal and distal anchors to collapse the wireforms of the device back into the catheter. The benign nature of the recapture sequence enables one to deploy a second device in the CS/GCV as needed.
Efficacy of the CARILLON™ Mitral Contour System™ has been tested in tachycardia-induced heart failure animal models. The benefit of this animal model is that it creates a representative ventricular pathology similar to the human condition where ventricular dilation, papillary muscle displacement, altered chordal tethering, and annular dilation all contribute to the development of FMR.
Results
Acute efficacy of a prototype percutaneous mitral annuloplasty device was initially demonstrated in an acute, open-chest, canine model of heart failure where the device was deployed under variable loading conditions, and the impact on haemodynamics was carefully assessed.10 Device deployment resulted in a decrease in mitral annulus diameter, the MR jet area, and the MR jet area/LA area ratio in both the presence and absence of phenylephrine. Acquisition of pressure volume loops confirmed that there were no significant changes in LV pressures, volumes, and systolic or diastolic function.
A follow-up chronic efficacy study was done with the same group at the Mayo Clinic where the device was placed percutaneously in a series of animals and efficacy was determined echocardiographically.10 A significant reduction in the MR jet area/LA area was documented between the heart failure baseline assessment (immediately prior to device deployment) and after 4 additional weeks of overdrive pacing (0.33±0.03 vs. 0.11±0.04).
The effect of the Cardiac Dimensions mitral annuloplasty device on haemodynamics was demonstrated in an acute ovine model of heart failure.11 Specifically, 9 sheep were implanted with the device that resulting in an average reduction of the mitral annular diameter of 22%. The resultant decrease in mitral regurgitation was associated with a decrease in the pulmonary capillary wedge pressure (26±3 vs. 18±3), a decrease in the pulmonary artery pressure (31±2 vs. 25±2), and an increase in cardiac output (3.4±0.3 vs. 4.3±0.4).
To assess the chronic effect of the Cardiac Dimensions device in an ovine tachycardia model, 14 sheep were paced until moderate MR developed, then 9 sheep were implanted with the mitral annuloplasty device, and the entire cohort was paced for an additional 28 days to compare the control group to the intervention group.12 All animals that received a device showed a significant reduction in pulmonary capillary wedge pressure and mitral valve regurgitation compared to the control group. In conjunction, significant improvements in plasma norepinephrine and brain natriuretic peptide were apparent.
Chronic safety was studied in a swine model where 15 animals were implanted with the CARILLON™ Mitral Contour System™, and haemodynamics and histology were studied at one, three, six, and twelve months.13 Mitral annular, septal-lateral diameter was acutely reduced 12±1% following the implant. No pressure gradient, diminished left ventricular ejection fraction, or coronary stenosis was found acutely or at follow-up. Device structural integrity was maintained for all devices at each follow-up interval, including 12 month follow-up. Furthermore, histological evaluation showed all parts of the device covered by fibrous connective tissue with complete re-endothelialisation by one month, little vascular injury, only mild inflammatory changes, and no venous stenosis.
Discussion
The goals that guide the development of a percutaneous mitral annuloplasty device entail maximizing efficacy and minimizing risk. In order to be clinically applicable, a novel device should be simple and reliable. Given the heterogeneous nature of the anatomy, a device should provide the flexibility to target a range of diverse pathology. For example, one should be able to adjust the degree of mitral annular reduction in order to provide therapy for patients with varying degrees of annular dilation and mitral regurgitation. One theoretical benefit of a percutaneous approach compared to a surgical approach is that haemodynamics can be varied real-time to simulate the increased haemodynamic load associated with exercise, and the physician can make adjustments to the annular repair as needed. Lastly, the ability to recapture a device provides additional flexibility and insurance in the event that rare events dictate reversal of a procedure. The device and procedure developed by Cardiac Dimensions are designed to provide all these features.
In addition to optimizing clinical usefulness, care must be taken to minimize the risk of adverse events. With the Cardiac Dimensions device, the risk of coronary artery compromise is minimized by the dual method of either positioning the device proximal to major coronary arteries, or real-time assessment of coronary flow, and either recapturing the device, or loosening tension in the event of coronary compromise. AV node compression is avoided by securing the proximal aspect of the device in the coronary sinus. The risk of tissue erosion and migration are minimized by virtue of the intimate relationship between the device and the tissue which facilitates endothelialization and prevents relative motion between the device and the adjacent tissue. The risk of CS occlusion is minimized by the helical nature of the anchor. The low profile of the device will likely enable pacing lead deployment through the helical anchors should clinical parameters indicate such adjunctive therapy. Lastly, the properties of nitinol can be leveraged to minimize the risk of thrombogenicity and mechanical fatigue failures. Both benchtop and pre-clinical studies have reinforced the safety profile of the CARILLON™ Mitral Contour System™.
Extensive preclinical studies have been performed in different heart failure animal models to characterize device efficacy. The value of a tachycardia-induced cardiomyopathy model is that the ventricular and annular dilation simulate the pathophysiology associated with FMR. Although it is difficult to model the spectrum of disease and the variability in pathology using animal modes, early insights into the potential efficacy of a device can be achieved. The consistent findings from two different animal models (i.e., canine and ovine), and the corroboration of associated haemodynamic variables, support the conclusion that the CARILLON™ Mitral Contour System™ significantly reduces mitral regurgitation in animal models of heart failure.
Carefully designed clinical trials are needed to understand the optimal utility of a percutaneous mitral annuloplasty device. Extensive research in the area of primary mitral regurgitation has shown that intervening prior to the development of significant ventricular deterioration is advantageous.14,15,16 Although there may be a clinical benefit to offering a percutaneous therapy to patients deemed high risk for surgery, there may be additional benefit in exploiting the minimally invasive nature of percutaneous mitral annuloplasty to intervene earlier in a disease course, and slow the remodeling often times associated with MR and volume overload. Eventual patient management will be guided by clinical trials which are designed to study a spectrum of different patient populations, and which assess the haemodynamic and functional benefits of percutaneous mitral annuloplasty.
Acknowledgements
Dr. Reuter is an employee of Cardiac Dimensions, and is a stockholder. The pre-clinical studies summarized in Results were funded by Cardiac Dimensions.

