KEYWORDS
Abstract
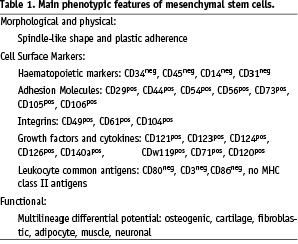
Mesenchymal stem cells (MSCs) are CD34 and CD45 negative, non haematopoietic stem/progenitor cells which are derived from the stromal fraction of bone marrow. As with other stem/progenitor cell subsets, the exact phenotype remains controversial. However they are characterised by adherence to tissue culture plastic without the requirement for a specialist substrate. Furthermore there are multiple surface antigens, such as adhesion molecules or integrins, which have been variously attributed to the MSC fraction and lead to further heterogeneity in their description. An operational definition has emerged that defines the MSC as a pluripotent cell with a differentiation capacity along with retaining multiple mesodermal lineages. Pre-clinical studies have indicated the benefit of both autologous and allogeneic transplantation in models of myocardial injury. A recently reported clinical study has suggested early safety of allogeneic cells in patients with acute myocardial infarction.
Introduction
Repair or regeneration of damaged myocardium represents a major challenge in the treatment of cardiovascular disease. Stem cell transplantation is being widely investigated as a potential therapy for cell death related heart diseases. Three distinct goals are required: first, regeneration with replacement of the myocyte loss; second, neovascularisation with restoration of blood supply; and third, attenuation of left ventricle (LV) remodelling. This is an ambitious goal, and several cell types are being tested. However, mesenchymal stem cells (MSCs) appear to demonstrate characteristics, such as homing capacity to myocardial injury, differentiation potential and immunological privilege, which may confer specific advantages over other stem/progenitor populations and make them the ideal cell for regenerative therapies.
Biology of mesenchymal stem cells
Bone marrow includes three distinct cell systems: haematopoietic, endothelial, and stromal. The stromal compartment supports the structure of the bone marrow, is responsible for the bone homoeostasis and contains a variety of cells including mesenchymal stem cells (MSCs). Like other stem cells, these MSCs are part of the nucleated cells and can be isolated from the Ficoll-gradient-isolated mononuclear fraction1-5. They are very rare, occurring at a frequency of ~ 1 cell per 104 nucleated cell, lack of a single common marker and are typically identified from the combination of morphological, phenotypic and functional properties. Current literature defines MSCs as spindle-like, CD34 and CD 45 negative stem cells which are able to adhere onto collagen-coated polysterene and which retain the capability to expand in the culture. They express also numerous surfaces as antigens such as adhesion molecules or integrins (Table 1). However there is not yet a widely accepted definition of MSCs. MSCs were originally described as having the SH2 and SH3 antibodies which are now classified as CD105 and CD73 respectively These markers were further extended to the presence of CD29, CD44 and CD90. Nevertheless, many groups contend that antigen expression alone is insufficient to distinguish MSCs from other populations. Note, the MSCs retain in vitro ability to express markers of the cells of mesodermal lineage, as well as neurons and astrocytes in response to growth factor stimulation.
A key characteristic which has recently been exploited in an early phase clinical trial is that MSCs appear to be immunologically privileged with MHC class I molecules but very low expression of MHC class II molecules in resting conditions. Interestingly though these MHC class molecules may be induced in response to growth factors, they do not interact with T cells presumably due to absence of the B-7 costimulatory molecules required for the e cellular immune response6.
Several investigators identified additional subpopulations carrying these main features of the MSCs, but yet being distinct from each other either because of multiple combinations of cell surface marker expression or due to various differentiation potential. For instance, Sca-1, c-kit, CD49a and STRO-1 antibodies have been used to isolate near homogeneous populations of mesenchymal stem cells7,8. However, the MSCs isolated using these antibodies differ from each other in their cardiogenic capacity, self-renewal ability, telomerase activity, and cell morphology. More recently, another subtype of the MSCs has been described having a wider transdifferation capacity; these so called multipotent adult progenitor cells (MAPCs)9 have been shown by some groups to retain both endothelial and cardiomyogenic potential. Other similar populations, include recently described human bone marrow-derived multipotent stem cells10 and marrow isolated adult multilineage inducible (MIAMI) stem cells11. These cell types merit further investigation assuming that consensus can be reached in the further standardisation of the isolation procedure and documentation of their cardiac regenerative potential.
Encouraging potential of MSC for cardiac repair: overview of pre-clinical and clinical applications
The ability of MSCs to differentiate into various cell types and to simultaneously secrete a number of growth factors makes them attractive for cellular therapy. Hypothetically, they may act in a dual way, affecting both cellular and trophic processes involved in the regenerative attempts4. While they may be used in the autologous cell transplantation, their comparative immune privilege makes them attractive from the perspective of the “off-the-shelf” use for broad clinical applications3,12.
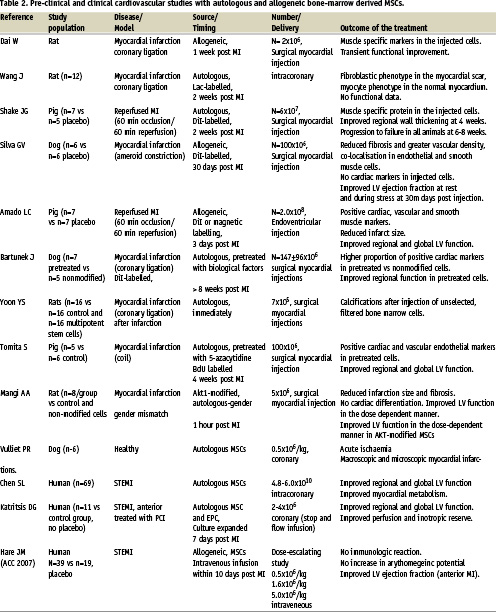
Preclinical studies have demonstrated that MSCs can differentiate at low frequencies into putative cardiac myocytes with the development of striated cells expressing a variety of cardiac specific markers13. However, this process appeared to occur at very low frequencies. Several studies have also demonstrated the beneficial effects of MSCs in experimental myocardial infarction (Table 2). In the rat or swine model of myocardial ischaemia/reperfusion injury, allogeneic mesench ymal bone marrow cells appeared to contribute to the neovascularisation and myogenesis, improving function at 30 days post infarction14-16. However, this effect led only temporarily into an improved cardiac performance, and most of the animals subsequently died from pump failure16. Furthermore, in a dog model of myocardial infarction, catheter based injection of the same cells led to a functional improvement parallel to increased capillary density despite absence of cardiac markers in the labelled cells17. Similar beneficial effects were observed in a pig study of acute anterior myocardial infarction, where MSC transfer resulted in improved regional and global LV function and reduced infarction size18. From the perspective of allogeneic use, it is also interesting to note that intravenous delivery of allogeneic cells immediately after reperfusion was associated with homing and long-term survival in the reperfused injured rat myocardium19, but that these findings were not reproduced in a large animal model of myocardial infarction in swine treated with allogeneic cells (J. Hill, unpublished data).
Nonetheless MSCs have entered the clinical arena. One of the first studies looked at the effects of autologous MSCs was studied in a small placebo-controlled randomised study of patients with acute myocardial infarction20. In this study, the culture expanded MSCs were extracted from the patient’s bone marrow and expanded then re-injected ~ 18 days after reperfusion via intracoronary route. The MSCs injection led to a substantial improvement in LV function and reduction of infarction size at 3 months, which persisted 6 months later. These preliminary findings, taken together with preferential homing to sites of injury and the unique immunologic features of MSCs have facilitated the rapid translation from these small scale animal studies placing us firmly into the clinical arena. Indeed such a study has recently been reported from the US which appears to have achieved its early primary objective of establishing the safety of the allogeneic MSCs in a clinical setting. The randomised, placebo-controlled trial, reported by Joshua Hare at the 2007 American College of Cardiology meeting included 53 patients, 34 of whom were infused with allogeneic MSCs intravenously within 10 days of an acute myocardial infarction, with 10 receiving placebo injection. There was a dose escalation component to this trial with three doses studied: 0.5 million, 1.5 million and 5 million cells/kg, each dose matched with the placebo. Early data suggested that allogeneic MSCs were neither associated with a higher incidence of adverse events, nor were any acute immunologic reactions observed. In contrast, there was provisional evidence of improved ejection fraction, more evident in anterior MI patients, although the study is underpowered to show this with subset analysis. These results seem to justify further study using the allogeneic MSCs, with the primary objective being to establish the efficacy on the surrogate endpoints together with broader safety data with regard to the allogeneic nature of the MSCs.
Controversies and future directions
Despite the attractive biological features of the MSCs and some positive experimental data as well as early promise in clinical trials, many controversies remain. Firstly, more insights are needed into the kinetics of coronary or systemic delivery to support this approach in the clinical setting. The recently reported clinical study has presented no evidence that intravenously delivered cells actually home to the area of myocardial infarction. In fact, MSCs following trypsinisation from tissue culture plastic typically have a larger size than mononuclear cell types and their coronary transfer has been associated with an additional risk upon myocardial necrosis in a dog model of myocardial infarction21. Likewise, systemic delivery could presumably result in lung entrapment of these cells with yet unknown biological or functional pneumological effects. However in the NIH and Osiris conducted safety studies, there were no deleterious effects seen with multiple doses of intravenously delivered allogeneic MSCs in swine, with respect to acute changes in oxygen saturation or inducible arrhythmia (J. Hill, unpublished).
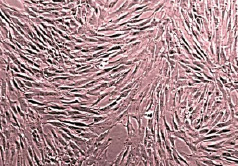

Figure 1. Bone marrow mesenchymal cells in culture. Upper panel: light microscopy; lower panel: cell labelled with GFP.
Secondly, there are several studies which resulted in controversial myocardial effects. They suggest that the injection of unmodified stem cells into the infarcted myocardium may be associated with other than the myocardial phenotypisation of these injected cells, leading to increased fibroblast formation or intramyocardial calcifications22-24. This may be related to differences in study design, experimental models or in the method of cell derivation. In this regard, it is worthy to note that in most of the studies that reported positive functional effects, cells were injected in the acute or subacute phase of the myocardial infarction. Such observations are also important from the perspectives of controversy regarding the ability of bone marrow progenitors to transdifferentiate in vivo into cardiomyocyte-like cells, likely given by the absence of appropriate inductive stimuli to recapitulate signalling necessary for cardiomyogenic specification in the chronically infarcted myocardium25,26. This led to the suggestion that alternative strategies involving coaxing the cells toward the cardiac lineage, or by addressing critical molecular pathways of cardiac differentiation, should be investigated27-30. Earlier, the strategy of ex vivo pre-transplantational cardiomyogenic specification was attempted with 5-azacytidine31,32. However, 5’-azacytidine as an inductive chemical compound is not suitable for clinical application due to its non specific epigenetic action. In contrast, cardiomyogenic differentiation potential in vitro was recently demonstrated by Li and colleagues by co-culturing MSCs with isolated neonatal cardiac myocytes33. Their findings support the concept that mimicking the cardiac micro-environment, in a manner similar to cardiac embryogenesis, might be a valid strategy to steer adult MSCs into the designated cardiac signalling, and to potentiate the functional effects after implantation into the injured myocardium25,27. This hypothesis is corroborated by our recent observations of superior biological and functional effects upon cardiac regeneration of chronically infarcted myocardium after myocardial injections of pre-specified MSCs as compared to unmodified cells34. Obviously, this concept requires further testing, including the need for further characterisation of the optimal growth factor cocktail, optimisation of the proliferative potential of the primed cells, their extent of differentiation and/or paracrine effects, and better cell survival. In these efforts, strategies including the biomechanical stimuli and biomedical engineering using various matrices in controlled conditions should be explored. Finally, it is also interesting to elaborate on the attractive hypothesis of Hare and colleagues, linking the exogenous cell delivery with endogenous cell tissue repair35. The Hare hypothesis, or the new “new paradigm”, is based on the assumption that the existing stem cell niche within the heart, i.e. cardiac residing stem cells, may be potentiated by exogenously delivered cells Exogenous cells may provide a new matrix for residing cells, and lead to multiple paracrine-mediated effects on survival, potentially differentiation and perfusion. It is possible that this mechanism is operational and may explain functional effects, despite the minimal cell survival and limited degree of cell differentiation.
In summary, the biological characteristics and unique properties of bone marrow-derived MSCs make them highly attractive for cardiac stem/progenitor therapies. In particular, the accumulated pre-clinical evidence appearing in some laboratories demonstrates their potential for attenuation of the negative cardiac remodelling that occurs post myocardial infarction. Ongoing clinical trials should establish both the long term safety and clinical efficacy of allogeneic MSCs in patients with recent myocardial infarction. Furthermore, novel strategies aimed at optimisation of myocardial effects in the setting of the chronic myocardial infraction should be explored.
Acknowledgements
Drs J. Bartunek, G. Heyndrickx, M. Vanderheyden and W. Wijns are members of a not-for-profit organisation which is a founding member of Cardio3. P. Willemsen and A. de Lavaraille are employees of Cardio3. Dr J. Hill received materials support from Osiris Therapeutics at National Institutes of Health, Bethesda. Comments and suggestions of Dr Ike W. Lee are greatly appreciated.

