The origin of the word “stent” has been greatly debated. Although often evoked, even by some older language services, it definitely has nothing to do with Charles Stent, a London dentist of the 19th century who, with the help of his sons, produced Stents mass which was meant to stabilise skin grafts. Stenting and stents are terms that were used already many hundred of years ago to describe the action of stiffening garments or stretching fishing nets across rivers1. The word ‘stenting’ is also synonymous with mining.
Scaffolding of hollow organs like the urinary track has been suggested thousands of years ago. It was only the logical consequence of observations that bougination of strictures in tubular biological structures like the urinary track or the gastro- intestinal organs usually was short lived. Our ancestral colleagues noticed that such strictures tended to recoil when the bougie was withdrawn.
The history of endoluminal scaffolding of blood vessels is considerably shorter. It was less than century ago that Alexis Carrel proposed the use of surgically placed man-made structures, like glass tubes, to maintain luminal patency of arteries. Carrel, who was only 39 years old and the first scientist working in the USA when he received the Nobel Prize was a man of many facets. His “certainty amounting to arrogance, that he was always right, his distain for those who disagreed with him” made him a figure of ambiguity1. By his biographers he has been quoted of saying as a young man: “What am I going to do, exists nowhere in the world. The researches I intend to undertake have been attempted by no one.” Richard J. Bing, who met Alexis Carrel first in Denmark and later developed a close working and personal relationship with him, called him “a strange, unusual and difficult personality”. On the other hand, Bing admitted that –to him– “Carrel was the kindest of men”2.
Carrel’s most admired achievements were about transplantation. Arterial scaffolding was a sideline. His publication on the permanent intubation of blood vessels in 1912 is probably the first reference in the literature regarding the use of what later became popular under the name of stent2.
Charles Dotter described early experiments in which metallic arterial scaffolds were placed in animal models3. However, because all those stents had thrombosed within 24 hours of implantation, research on human application of endo vascular stents was delayed by several decades.
All these details were totally unknown to me in 1984 when I exposed my very down to earth problem to a surgical colleague and friend, Dr. Velimir Mirkovitch at the University of Lausanne. My experience with angioplasty, which I started with minimal equipment received from Andreas Grüntzig himself in spring 1978, turned somewhat frustrating as soon as its limitations became apparent: a fairly high abrupt closure rate of about 5% and recurrence in the order of one in three. I contemplated an intraluminal scaffold to tack up dissections and prevent recoil (Figure 1).
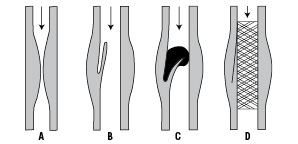
Figure 1. Intraluminal scaffolding.
Dr. Mirkovitch suggested to see the owner of a small firm in our neighbourhood (“Medinvent”) who, sparked by Åke Senning and a radiologist by the name of Zollikofer, had previous experience with some form of an intraluminal scaffold to prevent compression of large veins4. After many hours of brainstorming sessions with Christian Imbert, Christopher Knight and Hans Wallstén, I signed an agreement on a collaborative effort to develop a flexible low profile self-expanding endoluminal stent to which I had the right to attach my name. This stent became the first coronary stent to be used in humans (Figure 2).

Figure 2. Self expanding mesh stent.
The development of the self expanding mesh stent is a striking example of the way most technological breakthroughs see the light of day: the gathering of a small group of dedicated and enthusiastic people with a mission.
As soon as the delivery instrument had been perfected and tested in a simulator, animal trials were scheduled in Lausanne and Toulouse. In Lausanne, we chose the dog model, –nowadays almost unthinkable– sheep had the stent implanted in Toulouse. As the results of our dog implantations were extremely encouraging (contrary to the sheep trial in Toulouse), were extremely encouraging, the institutional review board of the University of Lausanne had no objection to authorize implantations in humans for three different indications:
– abrupt closure after angioplasty
– restenosis following previous angioplasty in coronary and peripheral vessels.
– stenosis of saphenous vein bypass grafts.
A number of implantations were performed in Toulouse and Lausanne, documenting the feasibility in man. The Lausanne anticoagulation regime to prevent thrombosis of the self-expanding mesh stent was a sophisticated balancing act between the risk of haemorrhagic and thrombotic complications. Valentine Fuster was instrumental in suggesting a succession of early heparin treatment followed by oral anticoagulation together with a combination of aspirin, persantin and sulfine pyrazone. Bleeding complications were frequent as no active puncture site management was available, and the customary compression consisted of a house officer leaning over the groin for an hour or so.5,6
The first ‘proof of concept’ and spectacular demonstration of the use of intraluminal scaffolds in the management of abrupt closure after angioplasty occurred in June 1986. During a live demonstration course at the University of Lausanne (to which, incidentally, Andreas Grüntzig had confirmed his attendance prior to his tragic aircraft accident), a female patient with double vessels coronary artery disease (proximal right coronary artery and proximal left anterior descending coronary artery) underwent balloon angioplasty performed by Barry Rutherford from Kansas City. Shortly after the intervention, while the operator was at lunch, the patient developed chest pain. She was moved back to the catheterisation suite with Professor Sadeghi, the head of cardiovascular surgery, eager and ready to go for emergency coronary bypass surgery. The right coronary artery was injected first and was found to be patent. Contrast injections into the left coronary artery, however, showed total occlusion of the previously dilated proximal segment of the left anterior descending coronary artery (Figure 3).
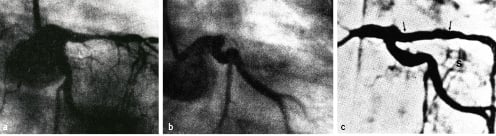
Figure 3. First bail-out stenting in 1986: a) LAD pre-PTCA, b) total occlusion post-PTCA, c) result at follow-up.
I briefly discussed the options with the patient in the presence of a somewhat impatient heart surgeon and we all agreed that we should attempt stenting. The consensus at that time between an interventional cardiologist and a heart surgeon to try to open the artery by implantation of a intra-vascular stent was a striking piece of collaboration and trust which changed the face of interventional cardiology, and, retrospectively, became the beginning the decline of bypass surgery.
Implantation of a self expanding mesh stent into the proximal left anterior descending coronary artery immediately solved the clinical problem, made the patient pain free and, apart from implantation of another stent into the restenotic lesion of the right coronary artery that had developed six weeks later, has caused event free survival up to now. The patient still feels great and has just celebrated her 50th wedding anniversary with her husband, who, incidentally, had bypass surgery only 13 years ago (“I was not as lucky as my wife”).
The reaction of the audience after this “first in man” intervention ranged from “why did you do this” to “extraordinary”. Visitors from all corners of the world came flocking in for the months to come to see, at a rather arbitrary rate due to the strict indications, stent implantations in coronary and peripheral vessels. One group was a delegation from the USA representing USCI, a firm implicated in interventional cardiology. The group was led by Gary Roubin, who, himself, together with Cesar Gianturco, was working on implantable scaffolds. Before departing the USCI representative asked me to make a guess on the likelihood of stents becoming clinical routine. In my youthful optimisms, without reflecting, I suggested that in my vision stents might be used in more than half of all coronary angioplasties within a decade. Retrospectively my prediction of a 60% stent rate by 1996 proved to be fairly precise: at the time of the “Stent Summit” in London which celebrated 10 years of the use of stents, and mainly due to the results of Benestent 17, stents were used in exactly 60% of all coronary angioplasty cases at our institution, the Royal Brompton Hospital. Now, two decades after the introduction of intraluminal stents, “stent-less” angioplasty has become a rarity, mainly due to the active suppression of smooth muscle cell migration and proliferation. By combining mechanical and biological principles transluminal angioplasty has literally replaced classic coronary artery bypass surgery in all but some rare, exceptional cases.
References

