Multicenter, historical controlled trial, sirolimus, drug-eluting stents, multivessel disease, percutaneous coronary intervention, coronary artery bypass surgery
Abstract
Aim: To determine the safety and effectiveness of CYPHER® sirolimus-eluting stent implantation in patients with multivessel disease; and to compare outcomes against the historical results of the two arms of the Arterial Revascularisation Therapies Study (ARTS I).
Methods and results: ARTS II is a 45 center, 607 patient single arm trial; the1-year outcomes were compared to the historical controls of the ARTS I trial, using conventional and Bayesian statistical methods. Patients were stratified by clinical site to ensure that at least one-third had 3-vessel disease to achieve the number of treatable lesions per patient comparable to ARTS I. Multivessel stenting was performed with sirolimus-eluting stents according to local institutional practice with the goal of achieving complete revascularisation.
The majority of patients (53.5%) had 3-vessel disease and diabetes was present in 26.2%. Mean stented length was 72.5mm, with 3.7 stents implanted per patient. The 1-year survival rate was 99.0%, the composite of death / stroke and MI-free survival was 96.9%, freedom from revascularisation was 91.5% and the composite endpoint of MACCE-free survival was 89.5% (the primary endpoint). Diabetic patients treated with sirolimus-eluting stents were more likely to undergo repeat revascularisation (RR 1.97, 95% CI 1.16 - 3.34) and experience a MACCE (RR 1.85, 95% CI 1.16 - 2.97) than non-diabetics at 1-year. In the unadjusted comparison with the historical control arms of ARTS-I-CABG and ARTS-I-PCI, the respective relative risks (RR) and associated 95% confidence intervals (CI) for the endpoints were: (1) freedom from repeat revascularisation RR 2.03 (1.23-3.34) and RR 0.44 (0.31-0.61) respectively; and (2) MACCE free survival RR 0.89 (0.65-1.23) and RR 0.39 (0.30-0.51) respectively.
Conclusion: The low incidence of MACCE and repeat revascularisation in ARTS II suggests that contemporary PCI with sirolimus-eluting stents is safe and efficacious for the treatment of multivessel coronary artery disease. Compared to the historical population of ARTS I, surgery still afforded a lower need for repeat revascularisation although overall MACCE rates in ARTS II approached the surgical results and were significantly better than bare stenting in ARTS I.
Introduction
Restenosis and the need for repeat revascularisation remain the major limitations of coronary angioplasty for patients with multivessel disease1-4. Drug-eluting stents have made a major impact on the effectiveness of percutaneous coronary interventions. The Cypher® sirolimus-eluting stent has been shown to significantly reduce restenosis and in-stent neointimal hyperplasia in patients with single vessel disease5,6.
Results from the randomized, multicenter Arterial Revascularisation Therapies Study (ARTS I) showed no significant difference in terms of death, stroke and myocardial infarction between the two groups, an overall 17% difference in repeat revascularisation in favour of surgery, and lower costs (US$ 2,973) at 12 months in favour of stenting1. This was confirmed by other similar randomized trials2,3. The RAVEL and SIRIUS trials with the sirolimus-eluting stent demonstrated a marked reduction in repeat revascularisation versus bare metal stents in patients with single vessel stenting5,6, as well as sustained efficacy and no evidence of late safety problems out to 3 years7. The RESEARCH single-centre registry demonstrated the feasibility, safety and effectiveness of sirolimus-eluting implantation in multivessel and complex patients8,9. Against this background, we performed the ARTS II trial with a similar inclusion criteria as ARTS I. The objective was to obtain information on the sirolimus-eluting stent in multivessel disease in a population whose baseline characteristics were to be at least of similar complexity and comparable to ARTS I10.
Methods
Study design
ARTS II is a multicenter, non-randomized, open labelled, stratified trial designed to evaluate sirolimus-eluting stent implantation in patients with multivessel disease; with the surgical group of ARTS I as an historical control8. After obtaining written informed consent, the patients were enrolled via a central telephone service. In order to obtain a population comparable to ARTS I, patients were stratified by clinical site in order to ensure the inclusion of at least 1/3 of patients with three-vessel disease. In addition, checks on the success of matching with the historical control were performed regularly during the conduct of the trial.
Patient selection
Patients were eligible for coronary revascularisation if they had either stable angina (Canadian Cardiovascular Society class I-IV), unstable angina (Braunwald class I-III B or C), or if they had silent ischemia and at least two new lesions located in different major epicardial vessels and / or their sidebranches (not including the left main coronary artery) that were potentially amenable to stent implantation. Patients were required to have multivessel disease with need for treatment of the left anterior descending (LAD) artery and at least 1 other significant lesion (>50% diameter stenosis) in another major epicardial coronary artery. The goal was to achieve complete revascularisation. One totally occluded major epicardial vessel or side branch could be included. The stenosis had to be amenable to stenting using a stent with a diameter of 2.5 to 3.5mm and length of 13 to 33mm, without restriction on the total implanted stent length. Decisions to place stents in lesions with bifurcations, fresh thrombus, calcification, diffuse disease, complex anatomy or stenting of side branches were left to the discretion of the operators.
Patients with previous coronary intervention, left main coronary disease, overt congestive heart failure or a left ventricular ejection fraction of less than 30 percent were excluded. Additional exclusion criteria included: history of a cerebrovascular accident, transmural myocardial infarction in the preceding week, severe hepatic or renal disease, neutropenia or thrombocytopenia, an intolerance or contraindication to acetylsalicylic acid or thienopyridines, the need for concomitant major surgery, and life-limiting major concomitant non-cardiac diseases.
Written, informed consent was obtained from each patient prior to enrolment. The study was approved by the ethics committee of each participating site.
Study objectives and endpoints
The primary objective of ARTS II is to compare the safety and effectiveness of coronary stent implantation using the sirolimus-eluting stents with that of surgery as observed in ARTS I. Endpoints are measured in terms of major adverse cardiac and cerebrovascular events (MACCE) at 1 year comprising all-cause death, any cerebrovascular event, non-fatal myocardial infarction, or any repeat revascularisation (either percutaneous or surgical).
The secondary objectives of this study are to compare the ARTS II patients to both arms of ARTS I with respect to: MACCE at 30 days, 6 months, 3 and 5 years; the combined end point death, myocardial infarction and stroke, and the itemized outcomes death, myocardial infarction, revascularisation procedure, stroke; resource use at 30 days and 1 year; cost effectiveness at 1 year, and quality of life at 6 months, and 1, 3, and 5 years.
End point definitions
Death from all causes were reported, and categorized as cardiac unless there was documentation to the contrary. Cerebrovascular events were divided into three main categories: stroke, transient ischemic attacks, and reversible ischemic neurologic deficits. In the first 7 days after the intervention, a definite diagnosis of myocardial infarction was made if there was documentation of new abnormal Q waves (according to the Minnesota code) and either a ratio of serum creatine kinase MB (CK-MB) isoenzyme to total cardiac enzyme that was greater than 0.1 or a CK-MB value that was 5 times the upper limit of normal1,11,12 Serum creatine kinase and CK-MB isoenzyme concentrations were measured 6, 12, and 18 hours after the intervention. Beginning 8 days after the intervention (the length of the hospital stay after surgery), either abnormal Q waves or enzymatic changes were sufficient for a diagnosis of myocardial infarction. This two-part method of defining myocardial infarction was developed for ARTS I to address the difficulty of diagnosing a myocardial infarction after surgery. A myocardial infarction was confirmed only after the relevant electrocardiograms had been analyzed by the electrocardiographic core laboratory and adjudicated by a clinical-events committee. All repeat revascularisation procedures were recorded.
End point measurement
In ARTS II, the procedure was performed within 48 hours after inclusion, while in ARTS I patients were randomized after informed consent had been obtained and then entered a waiting list, with 3 deaths in the ARTS I-CABG arm while on the waiting list. To compensate for the temporal difference since allocation between groups, events for the present report were counted from the time of the procedure for all three arms and not from the time of allocation as previously published1.
Statistical analysis
The sample size justification was based on the comparison of 1-year MACCE rates in the ARTS II patients and the ARTS I surgery patients for the primary end point. A MACCE-free survival rate of 90.9% was assumed in the ARTS II trial, requiring a sample size of 600 patients to guarantee a power of at least 90%.
Count variables are given as group rates and their matching 95% confidence interval. Continuous variables are given as group means, and the difference between groups presented with 95% confidence intervals. Time-to-event variables are presented as Kaplan-Meier curves. Safety data at 30 days and 1 year are presented as Kaplan-Meier estimates, with relative risks and 95% confidence intervals. Further analyses will be performed at 3 years with the final analysis at 5 years.
A separate multivariate regression analysis was performed to determine independent predictors of MACCE within the ARTS II population only. Clinically important baseline and procedural characteristics were tested on a per patient basis by univariate analysis to determine suitability for inclusion in the multivariate model. These variables were then entered into a stepwise logistic regression model with entry and stay criteria of 0.20 and 0.05 respectively.
A historical controlled trial design was used for this study. Unlike the gold standard of randomized controlled trials, historical controlled trials may differ in baseline and procedural characteristics, which potentially may affect outcome13. In order to overcome such differences, comparative statistical methodology, using both Bayesian and frequentist methodology, were used for the analysis of the trial10. Bayesian methods were used to test the additional hypothesis that the MACCE- free rate, adjusted for observed patient characteristics, is lower in ARTS II than in the two arms of ARTS I. A logistic regression model that incorporated the complexities of the design of each trial and the heterogeneity between patients within a trial was fit using Bayesian analysis (an approach well equipped for incorporating historical data in an analysis). An important feature of this approach is that it controls for unmeasured variables that predict MACCE in addition to observed predictors thereby facilitating comparisons between patients with different characteristics in different trials. However, in order to estimate the model, data from a second historical trial (RAVEL) needed to be included in the analysis5.
Results
Patients
Between February and November 2003, 607 patients from 45 participating centers were treated (Figure 1). Table 1 presents their baseline demographic and angiographic characteristics. Patients treated in ARTS II were a high risk population with a mean age of 63 years, and three quarters were male. Diabetes mellitus was present in 26% of patients and three vessel disease was present in the majority (54%). Seven patients in ARTS II did not receive any stents at the index procedure (4 underwent elective CABG, 1 required emergent CABG, 1 underwent percutaneous treatment 35 days later and 1 remained on medical therapy). The mean number of significant lesions per patient was 3.6±1.3 in ARTS II and 3.7±1.5 stents were implanted with average total stented length of 73±32 mm per patient. The mean duration of the procedure was 85 minutes and patients were hospitalized for 3.4 days post-procedurally.
In comparison to the ARTS I population, patients in ARTS II were significantly older, had a significantly higher percentage of patients with diabetes mellitus, hypertension, hypercholesterolemia and silent ischemia and a lower percentage of current smokers or had a history of prior myocardial infarction. ARTS II patients were also significantly more complex procedurally, with more three vessel disease (54% versus 30% in ARTS I-CABG and 27% in ARTS I-PCI) more significant lesions present (3.6±1.3 versus 2.8±1.0 in ARTS I-CABG and 2.8±1.0 in ARTS I-PCI). More stents and longer total stent lengths were implanted in ARTS II (3.7±1.5, 73 ± 32 mm) compared to ARTS I-PCI (2.8±1.3, 48 ± 22 mm respectively) At discharge, significantly more patients were prescribed medications for the secondary prevention of coronary artery disease in ARTS II compared to ARTS I.
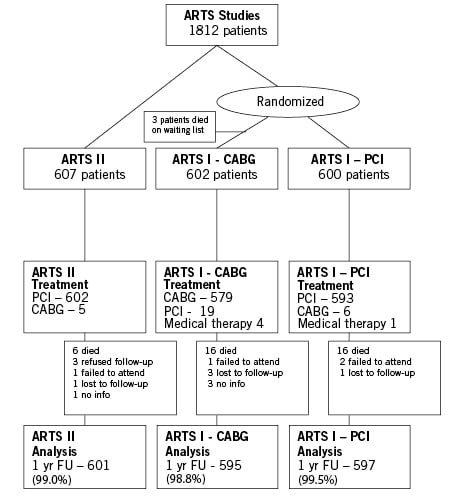
Figure 1. Flow chart of ARTS II and ARTS I.
Clinical outcomes
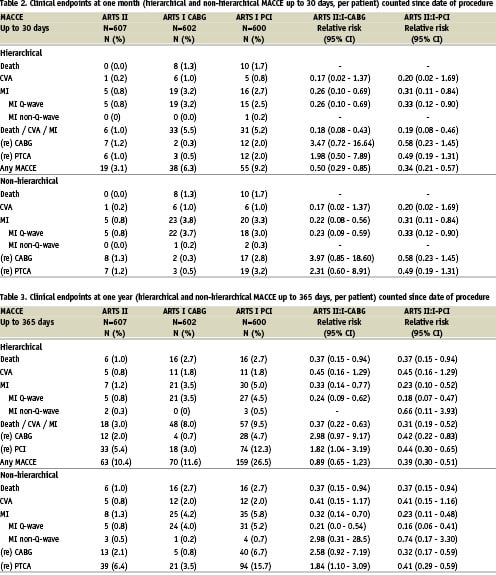
First 30 days (Table 2): In the ARTS II population, the 30-day composite MACCE rate was 3.1%. There were no deaths, 1 patient suffered a CVA and a Q-wave myocardial infarction occurred in 5 patients (0.8%); giving a combined endpoint of death, stroke or myocardial infarction of 1.0%. A repeat revascularisation via a percutaneous approach occurred in 6 patients (1.0%) and bypass surgery was required in 7 (1.2%). Thrombotic stent occlusions occurred in 0.8% of patients.
This 30-day MACCE rate was significantly lower than in ARTS I-CABG (6.3%, RR 0.50, 95% CI 0.29-0.85) and ARTS I-PCI (9.2%, RR 0.34, 95% CI 0.21-0.57). The lower incidence of death, stroke and myocardial infarction in ARTS II was less than in the ARTS I population (ARTS I-CABG 5.5%, RR 0.18, 95% CI 0.08-0.43) and ARTS I-PCI 5.2%, RR 0.19, 95% CI 0.08-0.46). The incidence of thrombotic stent occlusions was lower in ARTS II (0.8%) versus ARTS I-PCI (2.8%), p=0.009.
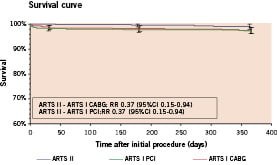
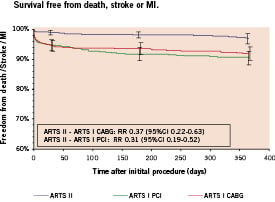
One year (Table 3 & Figures 2 and 3): At 1-year follow-up, the Kaplan-Meier estimate of the survival free of MACCE in the ARTS II trial was 89.5%. Six deaths occurred (1.0%), a further 5 (0.8%) suffered a stroke while 8 patients (1.3%) had a myocardial infarction, to give a composite death, stroke or myocardial infarction rate of 3.0%. Of the 6 deaths, four were adjudicated by the events committee to be of cardiac origin, although only one occurred suddenly and unexpectedly. The incidence of repeat revascularisation was 8.5% at one year with 39 patients (6.4%) who required a repeat percutaneous procedure, while 13 patients (2.1%) underwent bypass surgery in the follow-up period. The incidence of angiographically documented late stent occlusion (between 30 days and 1 year) was 0.3% (2 patients) in ARTS II.
The composite 1-year MACCE rate in ARTS II was in the same range as the ARTS I- CABG results (10.5% versus 11.7%, RR 0.89, 95% CI 0.65-1.23), with less events in the combined death, stroke or myocardial infarction endpoint (3.0% versus 8.0%, RR 0.37, 95% CI 0.22-0.63) balanced by a significantly higher need for repeat revascularisation in ARTS II (8.5% versus 4.2%, RR 2.03, 95% CI 1.23-3.34). Predictably, the 1-year MACCE rate in ARTS II was much lower than the ARTS I-PCI group (26.5%, RR 0.39, 95% CI 0.30-0.51), driven by the higher incidence of events in all measured endpoints. In particular, the repeat revascularisation rate in ARTS I-PCI was 21.3% (RR 0.44, 95% CI 0.31-0.61). Angiographically documented late stent thrombosis was not measured in ARTS I.
A
B
C
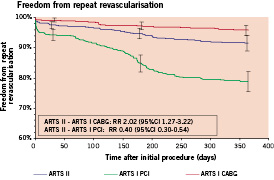
Figure 2. Kaplan-Meier curves out to 1 year in ARTS II and ARTS I: (A) Survival free; (B) Freedom from Death/CVA/MI; (C) Freedom from repeat revascularisation.
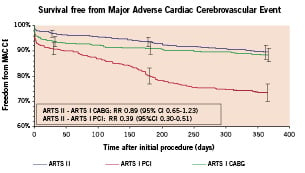
Figure 3. Kaplan-Meier curves out to 1 year in ARTS II and ARTS I: Freedom from Major Adverse Cardiac and Cerebral Events (MACCE).
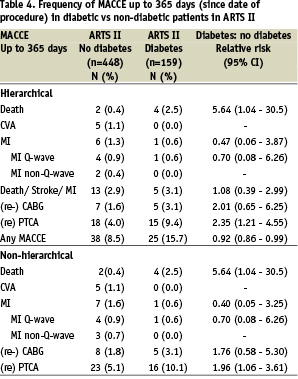
Diabetic versus non-diabetic patients (Table 4 and Figure 4)
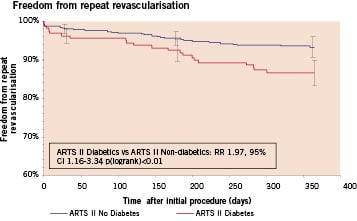
In the ARTS 2 population, diabetic patients had significantly higher one year MACCE rates by Kaplan Meier estimates compared to the non-diabetic population (15.8% versus 8.6%, RR 1.85, 95% CI 1.16-3.34, p (logrank) <0.01). This difference was driven by the increased need for repeat revascularisation in the diabetic population (13.4% versus 6.8%, RR 1.97, 95% CI 1.16-3.34, p (logrank) <0.01).
A
B
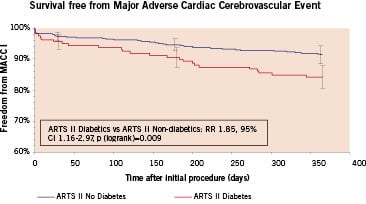
Figure 4. Kaplan-Meier curves out to 1 year in non-diabetic versus diabetic patients in the ARTS II arm: (A) Repeat revascularisation, (B) MACCE (Black represents No Diabetes, grey represents Diabetes).
Multivariate analysis (Table 5)
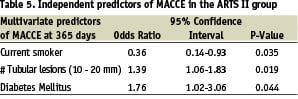
Multivariate analysis was performed on the ARTS II population to determine independent predictors of outcome in the sirolimus-eluting stent population. Variables significant in the univariate analysis were: treated lesions in the left circumflex, tubular lesions, diabetes mellitus, current smoker, number of lesions with a stenosis greater than 50%, Type B2/C lesions, age, and lesions with moderate to heavy calcification. Diabetes and the presence of tubular lesions were independently associated with adverse outcome, while patients who were smokers at the time of intervention were associated with a better outcome.
Bayesian statistical adjustment
Based on the logistic regression model fit using Bayesian methods, the adjusted MACCE rate was calculated to be 8.1±1.6% in ARTS II versus 13.1± 2.4% in ARTS I-CABG. To summarize the difference in treatment rates between the trial, the probability given the data from both trials that the MACCE rate in ARTS II is lower than the MACCE rate in ARTS I CABG was evaluated. The probability was 0.953, a value that would enable a significant difference at the 0.05-level to be claimed.
Discussion
The main findings of this study are: (1) contemporary percutaneous coronary intervention for complex multivessel disease using sirolimus-eluting stents was associated with low 1-year event rates for repeat revascularisation and overall MACCE, (2) the MACCE rate at 1-year was in the same range as that obtained in the historical bypass surgery arm of the ARTS I trial; (3) re-intervention rates in this contemporary group were still higher than the historical surgical cohort (4) the overall MACCE and especially re-intervention rates in this contemporary group were markedly reduced compared to the ARTS I-PCI arm.
The primary endpoint of this study, which was the composite endpoint of MACCE in ARTS II, was low, occurring in 10.5% of patients, and was within the same range as that seen with the historical surgical arm of ARTS I. A lower repeat revascularisation rate was noted in the surgically treated patients at one-year; this was balanced by a higher incidence of death / stroke and myocardial infarction.
In the secondary comparison between the two percutaneous arms, the significantly better MACCE rate with this contemporary PCI population treated with SES (ARTS II) compared to the bare stent PCI population treated in 1997-8 was primarily due to the lower reintervention rate; in spite of the higher baseline and procedural characteristic risk profile. Indeed, statistical analyses that adjusted for the independent predictors of outcome tended to find wider differences between the studies than unadjusted analyses. The incidence of stent thrombosis in the first month, as a surrogate for the acute safety profile of sirolimus-eluting stents in multivessel disease occurred in 0.8% despite increases in complexity and total stented length, compared with 2.8% in the bare stent arm of ARTS I-PCI confirming its safety in contemporary settings.
Within the ARTS II population, diabetic patients experienced a higher MACCE rate than non-diabetic patients, driven by an almost 2-fold increased need for repeat revascularisation in the first year, a finding confirmed by the multivariate analysis. Although event rates are markedly reduced compared to what has been seen with bare metal stents, diabetic patients continue to remain more resistant to the beneficial effects of sirolimus-eluting stents than non-diabetic patients. This phenomenon requires further investigation.
This present study was designed to determine whether the findings of the randomized trials with the sirolimus-eluting stent in single vessels could be extended to multivessel stenting. While a randomized trial design was always the preferred option, financial circumstances at the time of trial design dictated that only a single arm could be funded for such a study. This trial was thus seen as an intermediate step towards a full-fledged randomized trial with the original intention of a non-inferiority comparison using a historical control as stated in the protocol8.
The use of historical controls, as opposed to a randomized control is the subject of debate13,14. Randomized controlled trials, by virtue of their experimental design, provide a reliably unbiased estimate of treatment effects13. Historical controlled trials, on the other hand, suffer from a selection bias and of systemic differences in outcomes that may not be due to the treatment itself. Sacks found that this type of trial design over-estimated the treatment effect15. However, in two recent studies that compared both trial designs, no difference in treatment effect was seen16,17. Importantly, trials, both randomized and observational for a particular topic must be collectively and not individually examined to determine the accuracy in their results14. Hence, well designed historical controlled trials with appropriate matching, stratification and adjustment can be successfully used to guide the development of new trials.
Although arithmetically possible, the non-inferiority comparison between groups was replaced with descriptive comparisons due to the lack of randomisation coupled with concerns that the performance of the CABG group would have been better if assessed in the current environment as opposed to 5 years ago. The historical rates of ARTS I are thus viewed as standards against which the ARTS II study is compared.
The low event rates, including that of stent thrombosis noted in this study as compared to ARTS I may in part be explained by the more contemporary percutaneous coronary intervention methods used. Specifically, the use of lower profile and more easily deliverable devices may have resulted in less vessel trauma, a shorter procedure time; more comprehensive diseased vessel coverage with stents; and adjunctive glycoprotein IIb/IIIa inhibitor and/or intravascular ultrasound use may have contributed to the results. In addition, better secondary prevention practices, such as the more frequent use of lipid lowering agents, beta-blockers, and angiotensin-converting enzyme inhibitors may have contributed an added beneficial effect. Although the anti-restenotic effects of sirolimus-eluting stents are incontrovertible, one is unable to completely attribute the treatment effect to the device alone due to the non-randomized study design.
The role of complex multivessel stenting with drug-eluting stents will be addressed in two major randomized trials: FREEDOM and SYNTAX. The former will randomize 2400 patients with diabetes and multivessel disease to bypass surgery or drug-eluting stenting using approved devices, currently sirolimus-eluting and paclitaxel-eluting stents; while SYNTAX will randomize 1500 patients with 3-vessel or left-main disease to bypass surgery or paclitaxel-eluting stents. Nested preference registries are an added feature of both trials.
Study limitations
The results observed in this study require the following caveats in addition to those already mentioned. First, a five year time lag exists between the groups that are being compared. Both technology and medical practice have improved with time, as have surgical mortality rates18,19. Second, this study is non-randomized and thus the groups are not directly comparable, precluding a formal non-inferiority comparison. Furthermore, statistical adjustment was required to correct for the differences versus the historical control group, of which two methods of statistical analysis, namely frequentist and Bayesian, were performed in order to present robust results. Thirdly, while the protocol required that the lesions in ARTS II be potentially treatable by CABG, the absence of dialogue with the surgeons prior to intervention may have caused a selection bias. However, this is not obvious based on patients actually enrolled in the study since those enrolled in ARTS II were more complex than those enrolled in ARTS I. Finally, the results of the primary endpoint are reported for the one year timepoint only. While encouraging, the efficacy of sirolimus-eluting stents in the treatment of multivessel disease can only be definitively evaluated when long- term results become available in the future.
Acknowledgement
The co-authors would like to express their gratitude to Brian G. Firth, MD PhD in the drafting of the manuscript and conduct of the trial.
This study was supported by Cordis, a Johnson & Johnson company.
Appendix
Sponsor: Cordis, a Johnson & Johnson Company
Principal Investigator: Patrick W. Serruys, Rotterdam, The Netherlands
Steering Committee: Patrick W. Serruys, Rotterdam, The Netherlands (Chairman); A. Colombo, Milan, Italy; K. Dawkins, Southampton, UK; B. De Bruyne, Aalst, Belgium; C. Hamm, Bad Nauheim, Germany; C. Macaya, Madrid, Spain; M-C. Morice, Massy, France; G. Richardt, Lübeck, Germany; Macha Delattre, Director Clinical Research Europe, Cordis Corporation; A. Talen, Project Leader, Cordis Corporation; E. van Kleef, Clinical Trial Manager, Cardialysis BV, Rotterdam, The Netherlands.
Data Safety Monitoring Board: JGP Tijssen PhD, P Vranckx MD, FWA Verheugt MD
Endpoint Validation Committee: P van der Meer MD, F Kiemeneij MD, C Hanet MD
Data Coordination Center: Angiographic Core Laboratory: Cardialysis BV, Rotterdam, The Netherlands; ECG Core Laboratory: Cardialysis BV, Rotterdam, The Netherlands; Data Management Center: Cardialysis BV, Rotterdam, The Netherlands.
Site Monitoring: Cordis, a Johnson & Johnson Company
The following investigators participated in the ARTS II study (the numbers between brackets indicate the number of patients enrolled by each centre): MC Morice, MD, Institut Cardiovasculaire Paris Sud, Massy, France (23); C Macaya, MD, Hospital Clinico San Carlos, Madrid, Spain (22); PW Serruys MD, Thoraxcenter, Erasmus Medical Center, Rotterdam, The Netherlands (21); B De Bruyne MD, Onze Lieve Vrouwenziekenhuis, Aalst, Belgium (20); V Legrand, MD, CHU Sart Tilman, Liège, Belgium (20); G Richardt, MD, Segerberger Kliniken, Bad Segeberg, Germany (19); KH Kuck, MD, AK St.Georg, Hamburg, Germany (19); A Ramondo, MD, Azienda ospedaliera Padova, Padova, Italy (19); I Sheiban, MD, San Giovanni Battista Hospital, Torino, Italy (19); J Fajadet, MD, Clinique Pasteur, Toulouse, France (18); M Suttorp, MD, St. Antonius Ziekenhuis, Nieuwegein, The Netherlands (18); E Teiger, MD, CHU Henri Mondor, Paris, France (17); J Puel, MD, Hôpital Rangueil, Toulouse, France (17); S de Servi, MD, Ospedale Civile di Legnano, Legnano, Italy (17); KG Oldroyd, MD, Western Infirmary, Glasgow, UK (17); MCM Vrolix, MD, AZ St. Jan, Genk, Belgium (16); V Guetta, MD, Sheba Medical Center, Tel Ashomer, Israel (16); A Colombo, MD, Ospedalo San Raffaele, Milano, Italy (16); G Guagliumi, MD, A. O. Ospedali Riuniti di Bergamo, Bergamo, Italy (16); JW Jukema, MD, UMC Leiden, Leiden, The Netherlands (16); W von Scheidt, MD, Klinikum Augsburg, Augsburg, Germany (15); R Kornowski, MD, Rabin Medical Center, Petach Tikva, Israel (15); W Ruzyllo, MD, Institute of Cardiology, Warsaw, Poland (15); PG Steg, MD, Hôpital Bichat, Paris, France (14); MA De Belder, MD, James Cook University Hospital, Middlesbrough, UK (13); CW Hamm, MD, Kerckhof-Klinik, Bad Nauheim, Germany (13); A Betriu, MD, Hospital Clinic, Barcelona, Spain (13); KD Dawkins, MD, Southampton University Hospital, Southampton, UK (13); G Szurawitzki, MD, Elisabeth Krankenhaus, Essen, Germany (12); O Pachinger, MD, Universitätsklinik für Innere Medizin, Innsbruck, Austria (11); A Fontanelli, MD, San Bortolo Hospital, Vincenza, Italy (11); L Grip, MD, Salgrenska University Hospital, Goteborg, Sweden (11); K Endresen, MD, Riskhospital, Oslo, Norway (10); H te Riele, MD, Amphia Ziekenhuis, Breda, The Netherlands (9); I Kranjec, MD, Clinical Centre Ljubjana, Ljubljanan, Slovenia (9); J Dvorak, MD, University Hospital Vinohrady, Praha, Czech Republic (8); O Wittenberg, MD, Clinique les Franciscaines, Nîmes, France (8); K Virtanen, MD, Helsinki University Hospital, Helsinki, Finland (7); C Stefanadis, MD, Hippokration Hospital, Athens, Greece (7); V Voudris, MD, Onassis Cardiac Surgery Center, Athens, Greece (7); FW Amann, MD, Klinik am Park, Zurich, Switzerland (6); RD Levy, MD, South Manchester University Hospital, Manchester, UK (5); R Seabra-Gomes, MD, Hospital St. Cruz, Lisbon, Portugal (3); N Curzen/F Ordoubadi, MD, Manchester Royal Infirmary, Manchester, UK (3); I Horvath, MD, POTE Szigvgyogyaszati, Pecs, Hungary (3).
References

