Abstract
The ENTRATA™ transventricular aortic bioprosthesis (3F Therapeutics, Lake Forest, California) consists of a tubular structure assembled from three equal sections of equine pericardial material preserved in glutaraldehyde. The structure incorporates commissural tabs made from sections of pericardium that are anchored to the posts of the stent. The stent is manufactured from SMLS 316L vacuum melted extruded stainless steel tubing. Annealing of this stent allows compression and subsequent dilatation when within the aortic root. The bioprosthesis is delivered antegradely through the apical transventricular catheter system into the ventriculo-aortic junction. Fifteen acute, six sub-chronic and 6 chronic implantations of the device in swine have been done. Chronic implantations were done in miniature swine with the same delivery and deployment system, because subchronic implantations, (4-6 weeks) although proceeded without migration of the device from implant locus, without coronary blockage, thrombosis, dissection of the aorta or perivalvular leak, resulted in congestive heart failure due to excessive growth of the normal swine. The device shows promise as a transcatheter transventricular placement of an aortic bioprosthesis.
Introduction
The standard management of severe aortic stenosis has been valve replacement in an open heart procedure on cardiopulmonary bypass for the past 40 years. Percutaneous transcatheter dilatation of the aortic stenosis was commenced by Cribier et al.1-3 in the 1980s with only temporary suboptimal relief of critical obstruction. The procedure fell into disfavour except as a palliative procedure in non-surgical candidates or high-risk emergency situations. In 2002, Cribier commenced antegrade transseptal dilatation and deployment of an aortic bioprosthesis (Cribier-PVT-Edwards aortic bioprosthesis) for compassionate reasons4-6. Cribier used a 23mm prosthesis while Webb from St. Paul’s Hospital, University of British Columbia was successful in 2005 with a program using a 26 mm prosthesis by the retrograde arterial approach7.
The current study reports the transcather transventricular approach in a swine model of 3F Therapeutics ENTRATA™ aortic bioprosthesis system. This device was developed on the premise that replacement or repair of cardiac valves should be simplified. There is consideration that new devices, systems and methods should be developed without loss of patients welfare and safety.
Materials and methods
The ENTRATA™ transventricular aortic bioprosthesis (Figures 1 and 2) consists of a tubular structure assembled from three equal sections of equine pericardial material preserved in glutaraldehyde.

Figure 1. 3F Therapeutics ENTRATA™ Aortic Bioprosthesis
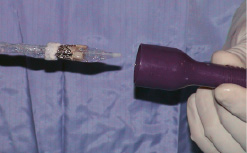
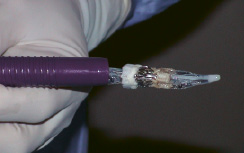
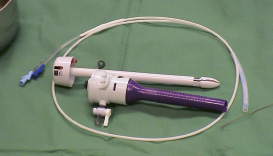
Figure 2. Crimped ENTRATA™ and Trocar
The structure incorporates commissural tabs made from sections of pericardium that are anchored to the posts of the stent. The stent is manufactured from SMLS 316L vacuum melted extruded stainless steel tubing. Annealing of this stent allows compression and subsequent dilatation when within the aortic root.
The device is crimped with a specially designed crimper (Figure 3) to a partially contracted state to fit within a 10 mm trocar.

Figure 3. Valve Crimper
The trocar is placed through the left ventricular apex to a ventricular cavity position. The device, inclusive of the crimped valve, is passed through the trocar. The bioprosthesis is delivered antegradely through the apical transventricular catheter system into the ventriculo-aortic junction. The native aortic valve is dilated by way of the apical transventricular delivery system. The positioning of the valvular prosthesis is facilitated by echocardiographic and fluoroscopic guidance. Valve competence is checked with injection of fluoroscopic contrast fluid.
The procedure was performed in the swine model with the objective to evaluate the safety and haemodynamic function of the implanted prosthetic valve. The safety of the device was determined by non-obstruction of coronary arteries, lack of migration, absence of thrombosis & thromboembolism, and complete seal of the aortic-ventricular annulus.
The swine procedure was performed with a 5 cm sternal incision to approach the cardiac apex. The delivery and deployment was conducted according to the above detailed protocol under heparin protection without cardiopulmonary bypass. The deployment was performed with the guidance of echocardiography and fluoroscopy (Figure 4).
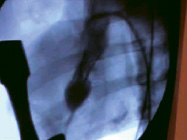
Figure 4. Device deployment and fluoroscopy
The measurement of the aortic root diameter was performed by transesophageal echocardiography to allow pre-surgical choice of valve size.
The above has been replicated fifteen times in acute procedures used for understanding of human factors engineering while training surgeons who later will use the device in the human clinical setting. The importance of navigation of the deployment system using various guiding tools such as intracardiac echocardiography (ICE) and cinefluoroscopy, to place the device in the appropriate locus or landing area, that is, the aorto-ventricular junction and demonstrate proper function by echocardiography and contrast fluoroscopy.
Six subcoronary implants in normal young domestic (55-65 Kg) swine were performed initially for subchronic implantations. We defined subchronic as four to six weeks. The experiment objectives were: 1) to demonstrate that proper positioning and delivery of the valve were feasible without migration or coronary blockage, 2) that the proper healing response would occur with complete sealing of the “annulus” of the aortic root, that is, without perivalvular leakage, and 3) with continued proper haemodynamic stability or valve function. Five of the six swine survived the planned period (1 month to 6 weeks). One died at two weeks due to an infection. In all other five swine, the valve was found to be properly positioned without coronary blockage, without any perivalvular leakage, and pre-sacrifice echocardiography showed proper valve function. Swine presented with all signs of congestive heart failure due to excessive rapid growth of all organs, that exclude the aortic valve bioprosthesis whose size is restricted by the stent.
The above experience, led us to attempt to minimize the growth and heart insufficiency problem by resorting to use of miniature swine (Sinclair-Yucatan), a special breed known to maintain a low weight through ageing. Six chronic implants (hoping for 150 days at terminus) were performed. Three swine were technical failures and did not survive the procedure. Of the remaining three, 2 survived the 20-week period, and one survived to 1 month before succumbing to an infection. The two 20 week survivors were found to be healthy and active at terminus. Preliminary necropsy reports a well-seated valve, without blockage of coronaries and complete sealing of the annular area. Valve leaflet function appeared normal.
Results
The surgical procedures were performed successfully and the animals were followed to final pathological examination at 4-6 weeks. There were no procedure or device related complications – no migration, no coronary blockage, no thrombosis, no dissection of the aorta, no perivalvular leakage and no pericardial effusion (Figure 5 and 6).
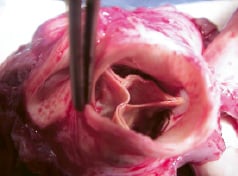
Figure 5. Aortic aspect of ENTRATA™ Aortic Bioprosthesis Explanted at 4 weeks
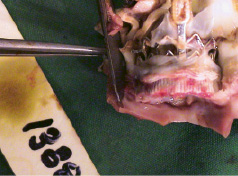
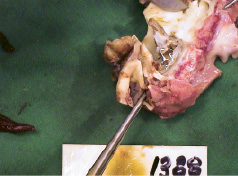
Figure 6. ENTRATA™ position at explant 4 weeks post-implantation
Discussion
The transventricular application of the 3F Therapeutics delivery system and deployment of the ENTRATA™ aortic bioprostheses was relatively simple in the swine model. Human implantation is anticipated as the next phase of following the successful animal series.
The flurry of reports on new techniques for transluminal placement of replacement aortic heart valves has shed light on the lack of a suitable animal model to validate and verify the concepts. We seemed to find the same obstacle initially and rather than use the ovine model others had tried unsuccessfully, we turned to the domestic swine. It became apparent that for the acute, one day experiment to learn the proper manipulation of the system we developed, this proved to be a very good model. For long-term studies (> 4wks) it became obvious that the problem was not only that of handling an animal with a very large mass, but that their rapid growth, with a heart and aorta growing also rapidly but a valve that could not, would result early in conditions of heart failure that could not support function through the term planned for the study. Thus, we resorted to the Sinclair-Yucatan swine strain that has been used in many pharmaceutical and surgical studies, and which allows for ageing without the extraordinary growth that is seen in the domestic strains. Although, only two survived to term, both showed excellent results without the development of congestive heart failure that plagued the domestic swine. This lead us to believe that this strain of swine may be the proper species for trans-cutaneous, trans-apical, cardiac valve implantation, or even trans-luminal cardiac valve, animal model development.
The human procedure will be performed through the left thoracotomy in the 5th intercostal space to gain access to the left ventricular apex (Figures 7 and 8).
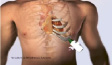
Figure 7. ENTRATA™ system approach through limited left thoracotomy at the 5th intercostal space
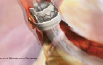
Figure 8. Illustration of ENTRATA™ system showing expanded prosthesis
Following placement of apical pledgeted suture, scalpel incision and trocar placement. Direct access of the crimped valvular device to the aortic annulus is through the trocar to the transventricular position. The size of the aortic annulus is determined pre-operatively by echocardiography. The valvular prosthesis is available in sizes 19-29. The anatomical landmarks of the native, diseased aortic valve, may be used in the clinical setting for positioning the bioprosthesis, aided by fluoroscopy and echocardiography. In the future, intra-cavity (ICE) and intravascular ultrasound (IVUS) will likely be adjuncts for future placement of intra-cavitary endovascular devices. The procedure can be protected from systemic embolization by a distal embolization protection device.
The major limitations of intravascular aortic bioprostheses are migration and peri-prosthetic leakage. The other limitations will be standard documented risks of bioprosthetic valves. The future role for transventricular aortic stented bioprosthesis, as well as antegrade and retrograde percutaneous delivered devices will be the safety and efficacy compared to standard surgically performed aortic valve replacement. Even if safety and efficacy does not match or surpass standard aortic valve replacement, there may remain a role in high risk patients with co-morbid disease or patients with accompanying life-limiting disease. The safety and efficacy will need to be determined in appropriately designed controlled trials in selected subsets of patients.
Both the valved stent and delivery system are being modified and being tested to ease the procedure and improve safety of the device.
The transventricular ENTRATA™ aortic bioprosthesis (Figure 9) shows remarkable promise by its design, delivery and deployment system and successful implantation in the animal model.

Figure 9. The ENABLE™ Aortic Bioprosthesis
The 3F Therapeutics ENABLE™ aortic bioprosthesis is a companion device designed for sutureless placement after native diseased aortic valve excision and annular debridement of calcification to enable shortened ischaemic and cardiopulmonary bypass times in high or moderately high-risk patients.

