Description
The AngioJet Rheolytic Thrombectomy (RT) System is a device for mechanical removal of fresh or semi-organized thrombus through percutaneous vascular access. Various versions of RT catheters have been developed and tailored for unique clinical applications.
History
Possis Corporation developed many industrial uses for water jet technology before applying the principles to miniaturized thrombectomy devices. Large industrial water jet systems were designed to cut difficult materials such as glass, titanium and stone. The special properties of water jet cutting, such as absence of significant heat or sparks, made it a preferred tool for cutting metals in flammable environments such as petroleum fields and refineries. In the early 1990s, Possis water jet devices were used to cut damaged piping from burning oil wells in Kuwait, preparing them for capping.
Possis Medical began developing the AngioJet RT System in 1989, initially for atherectomy applications, then with focus on thrombectomy applications1,2. Extensive preclinical testing has been performed3,4. The RT System is currently marketed with the following indications:
– Arterio-Venous Access indication: US 1996, CE mark 1997
– Coronary artery and saphenous vein graft indication: CE mark 1997, US 1999
– Peripheral arterial indication: CE Mark 1999, US 2000
The Company holds several patents for the unique catheter design. Ongoing research is focused in evaluating clinical applications for RT in the treatment of deep vein thrombosis and pulmonary embolism.
Technical specifications
The RT System consists of three components: a drive unit console, a disposable pump set, and a disposable catheter (Figure 1).

Figure 1. The AngioJet Rheolytic Thrombectomy System with its three components: the Drive Unit, the Pump set and the Thrombectomy Catheter.
The drive unit powers and controls the pulsatile pump and catheter, and provides feedback to the user for RT System set-up and operation. The pump set includes a high pressure pulsatile pump which is used to generate the flow necessary for the dissociation and evacuation of thrombus, an effluent bag for the collection and storage of thrombus debris, and associated tubing. Within the pump, pressurized saline is delivered to the catheter tip, creating a near-perfect vacuum (-600 mm Hg) for localized aspiration.
The Catheter is made up of 2 discrete pressure systems. The high-pressure system is contained within the stainless steel high-pressure hypotube that terminates distally in a closed loop (Figure 2a-c). The underside of the loop contains tiny jet orifices (3-7 total jets, depending on catheter model; orifice diameter is 50 um) that are directed retrograde back toward the catheter manifold (Figure 2d).

Figure 2. a. The closed loop end (arrow) of the stainless still hypotube at the distal end of the F105 catheter. Three high velocity retrograde-directed saline jets are clearly visible. The loop (arrows) visible at the distal tips of the Xpeedior (b) and Spiroflex (c) catheters. The improvements of the tip configuration, enhancing safety and deliverability, are clearly visible. d. Micro-photograph of the underside of the loop depicting one 50 um hole where the micro-jets originate.
The low-pressure system within the outflow lumen provides infusion pressure in Power Pulse Spray configuration (described below), and drives recirculation flow and exhaust flow in thrombectomy configuration (Figures 3a,b).
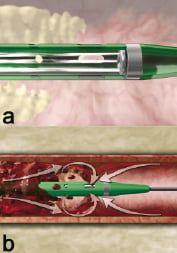
Figure 3. Rheolyic Thrombectomy System mechanism of action. a. High velocity retrograde-directed saline jets travel at 547 kilometres per hour creating a localized low pressure zone (-600 mm Hg) at the distal catheter tip (Bernoulli principle) for thrombus aspiration. b. Cross Stream windows create a low velocity fluid recirculation pattern for removal of mural thrombus. Thrombus is aspirated into the catheter where it is pulverized into small particles and evacuated from the body.
The high-pressure system is contained within the stainless steel hypotube and receives fluid from the Pump Set at 7,000 to 12,000 psi pressure. The Drive Unit has an automatic pressure-limiting cut-off at 12,000 psi. As the fluid exits the jet holes, the pressure is converted to velocity. The jets exit at approximately 16,000 cm/sec (maximum instantaneous velocity) and pressure drops to near zero absolute, which creates the suction action (Bernoulli Principle). The high velocity jets are contained entirely within the Catheter outflow shaft and do not come into contact with the vessel wall. Precisely configured ports are located in the distal outflow shaft to create a low velocity recirculation pattern around the catheter tip, referred to as Cross Stream® technology. The Cross Stream® recirculation facilitates the entrainment of mural thrombus.
Mechanism of action
Thrombectomy is accomplished by the introduction of a pressurized high velocity saline stream for thrombus entrainment at the catheter tip. Once in the pathway of the jets, thrombus is broken up into small particles, and the jets also provide the driving force for evacuation of the thrombus debris from the body through the catheter and associated tubing. Technical specifications for the various RT catheter models are shown in Table 1.
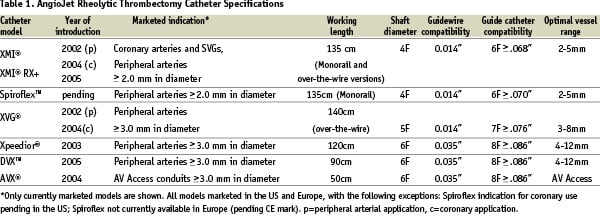
The evolution of RT catheters is shown in Figure 4.

Figure 4. Evolution of AngioJet Rheolytic Thrombectomy catheter. a. The F105 5Fr catheter, now obsolete. It was developed for peripheral arterial and AV access use. Note the jets directed into the exhaust lumen. b. The LF140 5Fr catheter, now obsolete. It incorporated a distal taper in tubing and a stainless steel cap attached to the hypotube loop to improve deliverability and increase safety for use in coronary arteries. c. The Xpeedior 6Fr catheter. It incorporates the Cross Stream technology (“windows” in distal shaft), a platinum marker band, and flexible tapered plastic tip; d. The XMI 4Fr catheter. It includes dual marker bands, smaller shaft size and enhanced tip taper for use in coronary arteries and rapid exchange (monorail) technology for quick and easy delivery. e. The Spiroflex 4Fr catheter. This rapid exchange catheter incorporates a spiral cut proximal shaft for enhanced handling and flexibility, extended-taper tip and a more flexible tip material.
Application of the RT Catheter for Power Pulse Spray
Power pulse spray infusion via the Xpeedior RT catheter is a recent innovation. A stopcock attached to the catheter manifold is used to close off the evacuation lumen; thus, the infused fluid is now directed out the distal catheter tip (Figure 5).
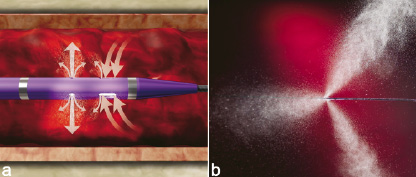
Figure 5. Power Pulse Spray with the Rheolytic Thrombectomy Catheter. a. Power Pulse Spray enables the infusion of a specified fluid (ie, thrombolytic drug) directly into the thrombus. b. The action of Power Pulse Spray ‘softens’ thrombus and prepares it for removal via the Rheolytic Thrombectomy Catheter.
The pressure of the pulsed spray is in the range of 20-30 psi at the catheter outer wall, and dissipates rapidly in blood. The technique may be used for directed infusion of physician-specified fluid such as a thrombolytic drug5. A Power Pulse Kit has been developed to provide the necessary materials and instructions for performing power pulse spray infusion. The kit is available in the US and market release in Europe is anticipated mid-2006.
Tips and Tricks/Technical considerations
– The XMI and Spiroflex RT catheters have a relatively low profile. Delivery over a 0.014” moderate support guidewire is recommended.
– The RT catheter is preferably delivered without predilatation; however, low pressure predilatation with an undersized balloon (1.5-2 mm in diameter) may be performed if necessary.
– For coronary applications, a recent study of RT in treatment in native coronary arteries described a technique for catheter activation at least 1cm proximal to the thrombus, to create a suction vortex prior to advancing the device in a proximal to distal manner6. This technique may be particularly useful in the setting of acute MI. Others have reported a technique for delivering the catheter distal to the thrombus, then operating in a pullback manner, especially for treatment in saphenous vein grafts that have large discrete thrombus with a proximal attachment point and a free-floating distal tail (ie, “rat tail thrombus”)7.
– Haemolysis may occur during RT, due to the shear forces of the high velocity jets. This is more frequently observed during extended catheter run times, such as in peripheral procedures. RT-induced haemolysis is not usually associated with clinical sequelae and is normalized within 24-48 hours post procedure. Care should be taken to observe the recommended run times specified in the catheter instructions for use, and to monitor haemolysis indicators in the blood, when warranted.
– Transient bradyarrhythmias have been observed during use of the RT catheter, particularly in the right coronary artery. It is recommended that a temporary pacing wire be placed to the right ventricle prior to initiating treatment. There have also been reports of preliminary experience using aminophylline pre-treatment to reduce or eliminate the requirement for temporary pacing during RT8,9.
Pre-clinical experience
In vitro testing has been performed to evaluate catheter effectiveness (clot removal rate) and safety (embolic particle generation and haemolysis), while animal studies assessed the haemodynamic, angiographic and histopathologic effects of RT operation, with results supporting safety and efficacy for clinical use3,4. Exploratory studies for neurovascular and intracerebral applications have also been performed10,11.
Clinical experience
The AngioJet System has been used in over 250,000 patients worldwide.
Coronary applications
The VeGAS randomized trial and registries establish the safety and effectiveness of RT in saphenous vein bypass grafts and native coronary arteries. The randomized pivotal trial was configured as a multicentre, 2-arm prospective study comparing immediate RT to intracoronary thrombolysis with urokinase (infusion for 6 to 30 hours), followed by definitive percutaneous treatment (12). The recently concluded multicentre randomized AIMI study showed no benefit for routine use of RT in treatment of ST-segment elevation MI (angiographic thrombus was not required in this study). However, a recent single-centre randomized study showed superior outcomes for patients treated with RT and direct stenting6, and provided the basis for the ongoing international multicentre randomized JETSTENT study to determine whether RT before direct infarct artery stenting results in improved reperfusion success in patients with acute ST-segment elevation myocardial infarction and angiographically evident thrombus.
Peripheral arterial applications
Prospective multicentre registry studies have been conducted, describing the angiographic and clinical outcome benefits of RT treatment for peripheral arterial thrombus13-15.

