Abstract
Aims: Significant technological improvements have made percutaneous aortic valve replacement (PAVR) simpler and safer, but the behaviour of the implanted valve over time remains unknown. We report the anatomo-pathological analysis of patients who died at different time intervals post PAVR with the CoreValve Revalving System.
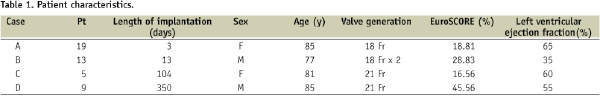
Methods and results: In our early experience, 21 patients underwent CoreValve implantation between 12/2005 and 02/2007. Among the 11 deaths at one year follow-up, four patients had an autopsy with macro and microscopic analysis. The device was divided in three parts during pathological assessment: the lower portion (area 1), the leaflets (area 2) and the upper part (area 3). The CoreValves were implanted for 3, 13, 104 and 350 days. Gross examination showed neointimal tissue covering most of the frame struts in contact with the aortic wall, but areas of high velocity blood flow were bare. Histopathology examination demonstrated fibrin deposition and inflammatory response early after valve implantation, followed by neointimal coverage with progressive regression of the inflammatory response over time.
Conclusions: While waiting for results of longer term PAVR echocardiographic follow-up, the anatomic assessment is encouraging with neointimal coverage of the native valve site and no excessive pannus formation occurring over the valve leaflets.
Introduction
Transcatheter aortic valve implantation is an expanding therapeutic option for a growing number of high-risk patients that are not considered to be suitable candidates for surgery1. Since 20022, about five thousand patients in Europe, Australia/New Zealand, Latin America and in North America have undergone this therapy with the Edwards SAPIEN valve® (Edwards Lifesciences, Irvine, CA, USA)3 and the CoreValve ReValving system® (CoreValve Inc., Irvine, CA, USA)4,5. Despite the large numbers of patients that have received this technology worldwide, little is known about the tissue response to this therapy. It is well known from pathological observation that soon after bioprosthetic valve implantation, deposition of fibrin, platelets and inflammatory cells occurs around the prosthetic ring which then forms a pannus6. By three months, this pannus is well formed around the valve ring and may even extend to the base of the valve leaflets.
In this article, we describe the pathologic analysis of four CoreValve devices removed from patients who died between day three and day 350, and attempt to further our understanding of the body’s response to the implantation of this device.
Methods
Patient description
In our early experience, between December 2005 and February 2007, 21 patients underwent percutaneous aortic valve replacement (PAVR) with two generations of the CoreValve device at the Montreal Heart Institute. All patients gave written, informed, consent. PAVR was approved for compassionate therapy by the Montreal Heart Institute Ethics Committee. The device was used either under special access by Health Canada or inside the Euro-Canadian efficacy and safety study with Health Canada sanctioned Investigational Testing Authorisation. Among the 11 deaths within one year of the procedure (three procedure-related deaths), four patients underwent an autopsy with macro and microscopic assessments. Another patient who died at day four had only macroscopic examination and has been described previously7.
Procedure description
The procedure was conducted as previously described in the literature8. In brief, through a femoral approach, the aortic valve was crossed in a retrograde manner with a straight wire using an Amplatz AL1 or AL2 diagnostic catheter (Cordis, Johnson and Johnson, Miami Lakes, FL,USA). Balloon valvuloplasty (BAV) was performed follow by the PAVR delivery catheter (21 Fr n=11 first patients, 18 Fr, n=10 last patients). The bioprosthesis was deployed under fluoroscopic guidance, aiming to deliver the ventricular extremity (inflow aspect) of the prosthesis 5 to 10 mm below the plane of the aortic valve annulus.
Adjunctive medication
For patients not currently under antiplatelet therapy, pretreatment with aspirin (80 mg/d, indefinitely) and clopidogrel (300 mg loading dose followed by 75 mg/d for at least six months) were administered the day before the procedure. Heparin was given during the procedure according to the patient’s weight to achieve an activated clotting time > 250 s.
Device description
The CoreValve aortic bioprosthesis is the third generation of a trileaflet porcine pericardial tissue valve, mounted and sutured in a self-expanding nitinol frame, which is 53 or 55 mm long, depending on valve size. The frame is divided in three distinct zones of radial force or hoop strength. The lower portion has high radial force to expand and exclude the calcified leaflets and to avoid recoil. This portion extends into the left ventricular outflow tract. The middle portion features high hoop strength and is the area of valvular attachment. The frame is constrained in order to avoid the coronary arteries. The upper portion of the frame has low radial force and is flared outward to make contact with the ascending aorta which serves to align the valve to blood flow. Of note, this device is implanted in the annulus but the valve portion is actually supra-annular.
The porcine pericardial tissue used is chemically processed very similar to that used in bioprosthetic heart valve industries. Phosphate buffered glutaraldehyde at low concentration is used to cross link porcine pericardial tissue. The effectiveness of chemical cross-linking treatment is monitored using industry standard of assessing shrink temperature (denaturation temperature), ninhydrin positive amino groups and resistance to enzymatic degradation; this tissue does not undergo supplemental anticalcification treatment.
Anatomo-pathological analysis
In our pathologic analysis, the CoreValve was divided in the three different parts corresponding to the zones described above. The lower part in the region of the native aortic valve or the inflow of the device was named area 1; the mid-portion, which carries the prosthetic aortic leaflets (functional valve area) was named area 2; and the upper portion of the device, or the outflow, corresponded to area 3 (Figure 1).
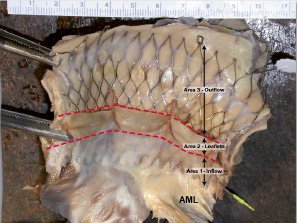
Figure 1. Heart opened in the left ventricular outflow showing CoreValve in place in situ. The valve assembly is divided into three regions which include: Area 1. Outflow area in the region of the native aortic valve corresponding to the inflow of the prosthesis; Area 2. Prosthetic aortic valve leaflets (between red lines); Area 3. Upper portion in region of ascending aorta, only the upper two rows are in contact with the aortic valve. AML: anterior mitral leaflet
Sections were taken from the various regions, including valve leaflets, and stained with hemtoxylin-eosin and movat pentachrome. Two of the valves were processed in plastic, including the nitinol frame with attached surrounding tissue, and stained with toludine blue and basic fuchsin.
Results
The four patients with autopsy died respectively at day 3, 13, 104 and 350. One autopsy (patient nr. 5) was performed outside our institution and we received only a fragment of heart tissue (ascending aorta with the CoreValve in place, left ventricle outflow tract including portion of the interventricular septum and the anterior mitral leaflet) already fixed in 10% formaldehyde. Patient characteristics are summarised in Table 1.
Case A – Explant at day 3
An 85 year-old lady (pt nr. 19) had a procedure-related death. Prosthetic valve placement was complicated 30 minutes following completion of the procedure by cardiac tamponade and arrest. Fifty cc of blood was evacuated from the pericardium, and the patient was rushed to the operating room where a perforation was identified in the aortic root close to the left main coronary take-off. The patient died of multiple organ failure on the third day. On gross examination (Figure 2), a thin layer of dull white endocardial tissue was seen on the interior surface of the device in area 1 where the prosthesis was in close contact with the native valve.
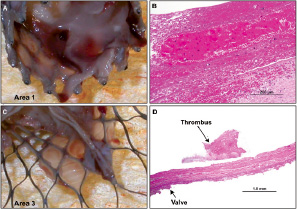
Figure 2. Case A: Views of the explanted CoreValve at day 3. A shows a thin layer of white film in area 1, likely representing fibrin rich thrombus in the region where the native valve would have been. In area 3 (C), the thin film focally covers some of the stent assembly. B and D are histologic sections taken from A and C. B shows focal presence of fibrin thrombus with interspersed red cells. D corresponds to the prosthetic valve (short arrow) with focal area of surface thrombus (long arrow).
The prosthetic leaflets were fully functional. There was no tissue seen in the proximal portion of the prosthesis in area 3. Small pieces of white dull tissue were visible on the inner surface near the valve suture areas on the frame. Microscopic examination revealed platelets, leukocytes and fibrin (Figure 2) with interspersed red cells surrounding the area 1 on the pericardial wrap of the valve frame.
Case B – Explant at day 13
A 77 year-old man (pt nr. 13) had an initial CoreValve prosthesis implanted too low, which resulted in an unacceptable degree of aortic regurgitation (AR). Implantation of a second CoreValve prosthesis (prosthesis in prosthesis) was performed as a bail-out. Transthoracic echocardiographic (TTE) assessment at day 9 showed a reduction of mean gradient from 40 mmHg to 16 mmHg and improvement of aortic valve area from 0.9 cm2 to 1.3 cm2. Nevertheless, this patient who had previously been operated for coronary artery bypass grafts, died of cardiac failure in the context of a pre-existing severe mitral regurgitation worsened by recent subendocardial antero-septal myocardial infarction involving the anterior papillary muscle.
On gross evaluation (Figures 3, 4), two valves were present, one inside the other without local complication.
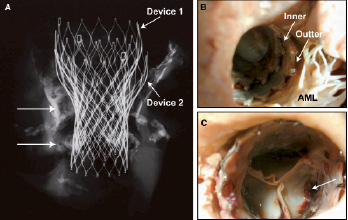
Figure 3 Case B: Radiograph and photographs of a double valve implant in place for 13 days. The radiograph (A) shows heavy calcification at the level of the native valves (arrows) and along the aortic wall (arrows). The gross photographs (B and C) show the inner surface of the outflow tract (area 1 or inflow of the device) and the ascending aorta (area 3). In B, in the area of the anterior mitral leaflet (AML) the double valve implant can be appreciated with mild thrombus covering the prosthetic valve. C shows the inner prosthetic valve (closed position) with mild red thrombus near the leaflet attachment sites (arrow).
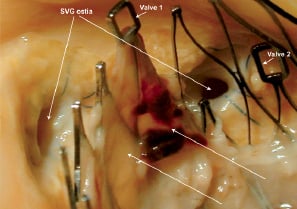
Figure 4. Case B: Gross photograph of the ascending aorta at the edge of the CoreValve devices (labelled Valve 1 and Valve 2). Note the functional saphenous vein grafts (SVG) ostium on the right are widely patent, and the metal frame of the CoreValve is bare whereas over the occluded saphenous vein graft ostium on the left (no flow), a white dull tissue was observed on the aortic valve frame (arrows).
In area 1, white dull tissue was visible on the interior surface covering both prosthesis. The first implant was placed too low in the outflow tract without any impedance of the anterior mitral leaflet, however, the valve was not well apposed to the outflow wall of the left ventricle. In area 2, the second valve was structurally normal with mild white dull tissue present in the aortic sinus. In area 3, the interior surface of the frame was also covered by white dull tissue. Interestingly, both areas in close contact with the aortic and ventricular walls were covered by the same tissue, and no thrombus was observed in the area of blood flow toward the coronary ostia, whereas over the occluded saphenous vein graft a white dull tissue was observed on the aortic valve frame (Figure 4).
Microscopic assessment (Figure 5) of the white tissue revealed fibrin intermingled with acute inflammatory cells, red blood cells and platelets in areas 1 and 3.
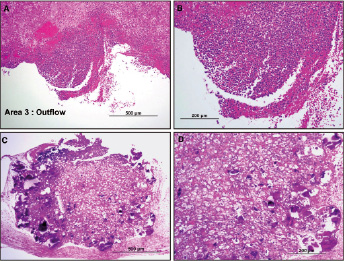
Figure 5. Case B: Histology sections of thrombus formation over the stent struts in area 3. A and B shows red blood cells (RBCs), fibrin and inflammatory cells. C and D demonstrates calcified debris trapped between the device and the ascending aorta surrounded by foamy macrophages and occasional giant cells, in a fibrin and red cell rich matrix.
In area 1, underneath the device and in the outflow tract a focal area composed of fibrin and red cell rich matrix with calcification was identified. There were focal foamy macrophages and occasional giant cells noted.
Case C – Explant at day 104
An 81 year-old woman (pt nr. 5) with moderate to severe mitral regurgitation prior to PAVR, died suddenly 104 days post-procedure. Most recent TTE at day 33 showed a well-functioning CoreValve with a mean aortic gradient at 8 mmHg (pre-PAVR: 54 mmHg) and a grade 1/4 perivalvular aortic regurgitation. On visual inspection at the autopsy (Figure 6), struts in area 1 were completely covered by a smooth glistening endocardial white tissue.
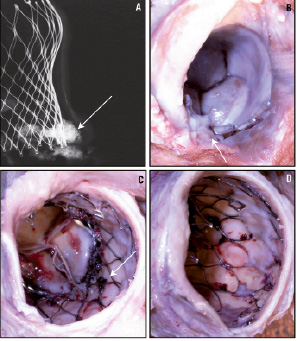
Figure 6. Case C: Radiograph and gross photographs of the CoreValve implant at 104 days. The radiograph (A) of aortic root with prosthetic valve assembly shows calcification at the level of the native valves and along the aortic wall (arrow). Gross photograph in area 1 (B) shows a calcified area inferior to the device (arrow) and CoreValve struts completely covered by tissue. Gross photographs in areas 2 (C) and 3 (D) show coverage of the distal spikes, but not the body of the device. Focal thrombus deposition is observed on the inner surface of the stents struts (arrow in C).
The leaflets (area 2) look structurally normal. In area 3, white intimal tissue was covering the distal spikes of the device, only at the areas which were in contact with the aortic wall. The portions of area 3 that were not in contact with the aortic wall were totally free of tissue coverage, which allowed normal blood flow into the coronary ostia.
On microscopic examination of area 1 (Figure 7), in the region of the native calcified aortic valve, the endocardial surface was covered by smooth muscle cells in a proteoglycan matrix, which extended to cover the pericardial skirt of the prosthesis and spread to the basal portion of the valve leaflets in area 2.
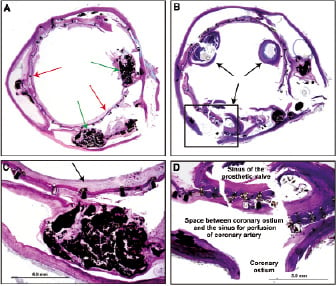
Figure 7. Case C: Histologic transverse sections of aorta with in situ prosthetic device in the region of the native valve (A) and at the level of the sinus of the prosthetic valve (B). In A the native leaflets are calcified (green arrows) and are excluded by the stent frame (red arrows). In C at higher magnification the calcified native leaflet is seen with neointima incorporating the stent frame (arrow). In D, the boxed area of the coronary ostium from B is highlighting the space between the prosthesis and ostium through which the coronary blood flow occurs.
A few inflammatory cells were identified and included macrophages, lymphocytes and giant cells (Figure 7). Also, an area of foreign body giant cell reaction was seen where cotton fibres got deposited secondary to the manipulation of the CoreValve at the time of valve loading before implantation. In area 3, there was neointimal tissue surrounding the frame struts in contact with the underlying aortic wall whereas in the region of the coronary sinus no neointimal coverage was observed on the frame.
Case D – Explant at day 350
An 85 year-old man (pt nr. 9), with history of coronary artery bypass grafts to the left anterior descending (LAD) and posterior descending arteries performed eight years prior to presentation with progressive aortic valve stenosis, died 350 days after CoreValve implantation in the context of renal failure and respiratory distress. The autopsy revealed a well functioning bioprosthetic valve and underlying cardiac amyloidosis. TTE assessment at 326 days showed a mean gradient of 15 mmHg (43 mmHg at baseline, 9 mmHg at six months) and an aortic valvular area of 1.2 cm2 (0.7 cm2 at baseline, 1.6 cm2 at six months).
By gross examination (Figure 8), in area 1, frame struts and pericardium were completely covered by a glistening white endocardial tissue.
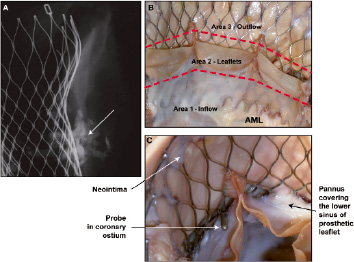
Figure 8. Case D: Radiograph and gross photographs of the valve implant at 350 days. The radiograph (A) of aortic root with prosthetic valve assembly shows heavy calcification at the level of the native valves and along the aortic wall (arrow). Gross photograph B shows the three areas of the valve and pannus growth covering the lower sinus of the prosthetic leaflet (AML = anterior mitral leaflet). Higher magnification of the prosthetic valve and the metal frame (C) shows a probe tip in the coronary ostium (arrow), pannus in the valve sinus near the ostium (arrow) and neointima covering part of the CoreValve frame in area 3 (arrow).
In area 2, the white tissue was seen creeping over the valve sinus, both over the ventricular and aortic side. The left internal mammary artery to the LAD was patent and severe left proximal calcific coronary artery disease was also noted. In area 3, the frame struts in contact with the aortic wall were totally covered by intimal glistening white tissue, but the upper and lower portion of the frame not in contact with the vessel wall remained totally bare (Figure 8). Microscopic examination showed all areas with glistening white tissue consisting of smooth muscle cells within proteoglycan collagenous tissue with endothelial covering (Figure 9).
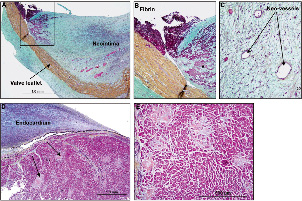
Figure 9. Case D: Histology sections from the 350-day implant. A shows prosthetic leaflets with organised neointimal pannus covering both surfaces of the leaflet in the area of the commissure. B and C are higher magnifications of the organising thrombus and neovascularisation of the pannus, respectively. D and E are histologic sections of the myocardium near the outflow tract showing endocardial thickening and focal infiltration of the myocardium by amyloid deposits (arrows).
In area 1, there were no signs of inflammation and the surrounding cardiac myocytes were focally separated and surrounded by amyloid deposits, consistent with senile amyloidosis (Figure 9). In area 2, on both sides of the prosthetic porcine pericardial leaflets there was presence of pannus, which consisted of smooth muscle cells in a proteoglycan-collagenous matrix (Figure 10). Organising granulation tissue was observed focally around the strut frame as well as smooth muscle cells in proteoglycan rich matrix (Figure 10).
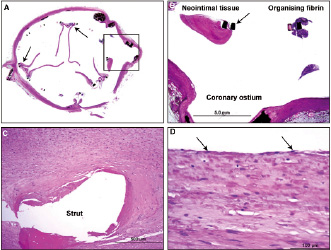
Figure 10. Case 4D: histology section from the 350-day implant. A. Transverse section of the aorta and valve assembly at the level of the prosthetic leaflet commissures (arrows). B. High power view of the boxed area from A showing patent coronary ostium and sinus space between stent frame (arrow) and ostium. Note fibrin deposition and neointimal tissue around stent frame focally. C. High power view from area 3 around the stent frame showing neointimal tissue consisting of smooth muscle cells in a proteoglycan collagen matrix. D. High power magnification of the neointima around the stent frame showing coverage of the neointima by endothelial cells (arrows).
The self-expanding frame of the CoreValve prosthesis adapted itself adequately to the four different anatomies of these patients. The in vivo valve area was over 1.3 cm2 in the four patients with only trace of paravalvular aortic regurgitation. Depth of the implantation needed to be corrected in patient nr. 2 with a second prosthesis, sealing the para-valvular leak. Even in this extreme low position, the integrity of anterior mitral leaflet was preserved, confirming the significant anatomic distance between the 2 annuli in human.
Discussion
We demonstrate the chronological integration process of this specific porcine pericardium bioprosthesis. Indeed, valves were implanted for less than two weeks in two patients and for three months and one year, respectively in the two other patients; thus allowing pathologic assessments at early and late time-points for the CoreValve ReValving system.
The valve incorporation process starts with early fibrin deposition associated with an inflammatory response and some foreign body reaction. Following three months, fibrin is replaced by smooth muscle cells and endothelial cells. There was evidence of focal areas of persistent residual inflammation. Over time, neointimal tissue became less cellular but more fibrotic as proteoglycans and type III collagen were replaced with type I collagen.
The gross examination allowed us to appreciate the distribution of the neointima which conformed to the design of the CoreValve ReValving system. Neointimal tissue covered most of the frame struts in contact with the aortic wall, but areas of high velocity blood flow (i.e., patent saphenous vein graft or native coronary ostia) were not enveloped by the neointimal tissue. The neointimal tissue also spread to limited portions of the CoreValve leaflets closest to the valve attachment sites. However, this was noted in the patient with senile amyloidosis. It is possible that amyloidosis could have enhanced tissue proliferation9 such that the extent of response was excessive and is applicable only to this patient. In fact, in the case described by Linke,10 without amyloidosis there was no excessive pannus after more than 400 days of implantation. We definitively suspect that the cardiac amyloidosis played a role in this pannus extension in this patient.
Nevertheless, in comparison with most surgical bioprosthesis, which are protected by a ring preventing tissue invasion, the porcine pericardial valve sutured to the nitinol frame may be more vulnerable to neo-intimal invasion. Under adverse physiological conditions, such tissue proliferation could possibly lead to more rapid structural valve deterioration than following surgical bioprosthesis valve implantation, but this is highly speculative. In the case of our patient, echocardiographic assessment 15 days before his death showed a well functioning valve, with a slight increase in mean gradient.
This tissue proliferation could also be seen as a favourable phenomenon: a kind of chimerisation of the valve leaflets which could slow down the structural valve deterioration process. In the near future, longer post-PAVR clinical follow-up, echocardiographic and anatomic assessment will provide data on how and when these transcatheter porcine pericardial bioprosthesis are likely to deteriorate over time10. This approach will improve our understanding of the bioprosthesis integration process and of the possible neointimal tissue extension on the leaflets.
Valve-in-valve implantation occurred in one of our patients. During the visual examination, we could appreciate the adequate interaction of the two devices and fibrin coverage around both inner luminal surface of the prosthesis. Even if one of the two valves was too low, there was still no interaction with the anterior mitral leaflet. Indeed, the landing zone between the aortic annulus and the mitral leaflet was large enough to not interfere with the anterior mitral leaflet. Observations in human autopsy specimens indicated that there is approximately 1.0-1.5 cm between the aortic annulus and the anterior mitral valve leaflet known as the “aortic-mitral fibrous continuity” i.e. a virtual line joining the basal attachments of the aortic leaflets to the “hinge” point of the anterior mitral valve leaflet
In all four of our cases, but specifically critical in the valve explanted at 350 days, the nitinol frame was intact with no evidence of fracture. In comparison, in percutaneous pulmonary valve replacement the highest incidence of device fractures happened during the first 400 days (17% rate of platinum-iridium stent fracture), and is likely related to the different material being used for the frame of the valve11 as well as the differing anatomical compliance model encountered. This complication should not happen with the nitinol frame in the aortic position as dynamic (movement) is different from the pulmonary position, even though the blood pressure is higher. Also at the level of the prosthetic leaflets, gross examination excluded cuspal tears or perforations and microscopic evaluation revealed no evidence of early leaflet degeneration or connective tissue failure i.e., fragmentation of collagen, microscopic calcific deposits.
As a result of the new information from the examination of these four valves, we confirm that clopidogrel should be advocated for at least the first three months during the early period of the incorporation phase of the valve frame. Thereafter, when the neointimal layers with smooth muscle cells and coverage of the lumen by endothelial cells has occurred, it may be safe to discontinue the clopidogrel.
Conclusion
Histopathology examination of our four valves at autopsy showed two different time-points in the integration of the CoreValve ReValving system: fibrin deposition and inflammatory response early after valve implantation followed by late neointimal coverage with progressive regression of the inflammatory response over time. Valve integration into the annulus and outflow tract is adequate and the underlying valve does not interfere with the normal functioning of the valve prosthesis. While we await for the results of longer term PAVR clinical follow-up with echocardiographic assessment, the anatomic assessment is encouraging with neointimal coverage of the site of the native valve and no excessive pannus formation occurring over the valve leaflets. Percutaneous aortic valve replacement is a rapidly developing field and may represent a solution for high risk surgical patients with aortic valve dysfunction, either with native aortic valve stenosis or degenerated of bioprosthetic valves12.

