Abstract
Aims: Sirolimus-eluting stents (SES) have been shown during short follow-up periods to be effective for treatment of in-stent restenosis (ISR). This study evaluated the 30-months clinical efficacy after SES for treatment of ISR in comparison with intracoronary radiation therapy (IRT).
Method and results: Seventy-two consecutive ISR lesions in native coronary arteries (<30 mm lesion length, reference diameter <3.5 mm) of 72 patients were treated with SES. SES were used in 16 lesions after failed IRT and in 56 lesions for first time ISR. Seventy-two patients with 72 lesions from a prospective registry of 141 patients treated with IRT (β-radiation) were matched for diabetes, reference vessel diameter, lesion length, and pattern of ISR to present the control group. At 6-months in-stent late loss was 0.29±0.48 vs 0.53±0.63 mm for the SES group compared to the IRT group (p=0.025). Target lesion revascularisation (TLR) at 12 month follow-up was performed in 7 lesions (10%) after SES and in 17 lesions (24%) after IRT (P=0.025). TLR rate at 30-months was 13% in the SES group vs 32% in the IRT group (P=0.008). MACE (death, myocardial infarction, target lesion revascularisation) at 30-months was observed in 13 patients (18%) in the SES group and in 25 patients (35%) in the IRT group (P=0.024). Considering only patients treated with SES for first-time ISR, TLR rate was 3.5% at 30-months. In the 16 patients treated with SES after failed IRT TLR rate was 44% at 30-months.
Conclusions: Thirty month clinical follow-up of patients treated with SES for first time ISR is favourable compared to follow-up after IRT. However, use of SES after IRT failure is associated with a high rate of recurrent and potentially late treatment failure.
Introduction
Intracoronary radiation therapy (IRT) has been proven to be an effective catheter-based modality to lower recurrent in-stent restenosis (ISR) compared to mechanical treatment modalities during follow-up periods of 6 to 9 months1-3. However, there are several limitations to IRT. Late progression of intimal hyperplasia beyond a 12 month follow-up period may result in delayed restenosis and need for recurrent target lesion revascularisation (TLR)4,5. Late thrombosis, edge-effects and aneurysm formation are other draw-backs which hamper its long-term safety and efficacy6,7. Subsequently, an erosion of therapeutic effectiveness during longer follow-up periods has been described8. Recurrent restenosis after IRT represents a therapeutic challenge. Even after repeat IRT recurrent restenosis has been reported to occur in 25% of cases9.
Drug-eluting stents (DES) have been found in randomised trials to result in superior clinical and angiographic results during follow-up periods of up to 9 months after treatment of ISR in comparison with IRT10,11. The potential for late attrition of treatment effects after implantation of DES for ISR similar to those observed after IRT remains a concern. Whether the inhibitory effect on in-stent intimal hyperplasia and subsequently the reduction of recurrent ISR sustains more than 12 months after DES implantation has not been evaluated yet. There are also no data regarding the risk of late stent thrombosis after DES for ISR and the long-term results following the use of DES for recurrent in-stent restenosis after failed IRT.
This study aimed to evaluate (1) 30-month clinical efficacy and safety after sirolimus-eluting stents (SES) for treatment of ISR in comparison with IRT using β-radiation in a matched-pair analysis, (2) the effectiveness of SES for the treatment of in-stent restenosis after failed IRT in comparison with SES for treatment of first time
in-stent restenosis.
Methods
Patients and lesion
One hundred forty-four patients with symptomatic ISR lesions (>50% diameter stenosis) in a native coronary vessel were treated at the two participating centres using either SES or IRT. Follow-up angiography was performed routinely after treatment of complex ISR lesions requiring SES or IRT at both institutions.
The patient inclusion process for this study is given in Figure 1. Seventy-two consecutive patients with 72 consecutive ISR lesions (<30 mm lesion length, reference diameter <3.5 mm) were treated with SES between July 2002 and December 2003 and were included in a prospective registry. Follow-up angiography at an average of 6.3±1.2 months was available in 58 (81%) lesions. In all patients a 30-month clinical follow-up was obtained.
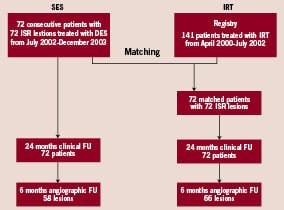
Figure 1. Flow chart demonstrating the patient inclusion into the two treatment groups.
Seventy-two patients with 72 lesions treated with IRT represented the control group. This group was recruited from a prospective registry of 141 consecutive patients (174 lesions) undergoing β-radiation therapy for ISR between April 2000 and July 2002 at the University Hospital in Aachen. Patients and lesions were matched with the SES group considering the following parameters in sequential selection: 1) diabetes; 2) reference vessel diameter (±0.2 mm); 3) lesion length (±2.0 mm); and 4) pattern of ISR according to the classification by Mehran et al12.
In the matching process only one lesion per patient was included. All other clinical and angiographic variables were blinded for matching purposes. Sixty-six patients (92%) of those 72 patients/lesions in the IRT group used as the control group underwent angiographic follow-up at an average of 6.4±1.5 months, while clinical follow-up at 30 months could be obtained in all patients.
Procedure
During the procedure heparin was administered according to standard practice. Aspirin (100 mg/day) and clopidogrel (300 mg loading dose) were started before the procedure. Subsequently, clopidogrel (75 mg/day) was administered for 6 months in the SES group and for 12 months in the IRT group in addition to aspirin.
Direct stenting was performed in 51% of lesions treated with SES. A second wire was placed within the vessel to prevent balloon slip within the restenotic lesion in case of predilatation before placement of a SES. Pre-dilatation was performed with a balloon 0.5 mm smaller than the subsequently implanted stent. Implantation of a SES with a length longer than the initial balloon length was encouraged. Sirolimus-eluting stents (Cypher™, Cordis Corporation, Johnson & Johnson, U.S., 140 µg sirolimus/cm2 metal surface area) in lengths of 8, 13, 18 and 28 mm and diameters of 2.5, 2.75, 3.0 and 3.5 mm were used.
For IRT a 5F delivery catheter and a 60 mm source train of strontium-90 seeds (non-centred β-emitter, 90Sr Novoste Europe SA/NV Brussels, Belgium) was used. Balloon angioplasty was the method to implement lumen enlargement in the IRT group. To prevent balloon slip within the restenotic lesion a second wire was placed within the vessel. Only in case of dissection or remaining stenosis implantation of additional stents was performed before IRT.
In-hospital and 30-month clinical follow-up
Procedural success was defined as a <30% final diameter stenosis in the treated lesion and the absence of major clinical complications (in-hospital death, Q-wave myocardial infarction, or emergency coronary bypass surgery). All patients were monitored for 30 months after the procedure for any major adverse cardiac event (MACE), defined as death, myocardial infarction, or need for repeat target lesion revascularisation (TLR) with regular telephone contact at 12, 24 and 30 months. Death was defined as all-cause mortality. Q- and non-Q-wave myocardial infarction were defined as a total creatine kinase (CK) elevation > 2 times normal or CK-MB > 20 ng/mL (upper limit 6.5 ng/mL) with or without new pathological Q-waves in 2 or more contiguous leads. Subacute stent thrombosis was defined as stent thrombosis occurring between 24 h and 30 days after the index procedure. Late total occlusion was defined as angiographically documented total occlusion > 30 days after the index procedure6. Baseline clinical demographics, in-hospital complications and the occurrence of MACE during follow-up were verified by independent hospital chart review and source documentation.
Quantitative coronary angiography
Quantitative angiographic analysis was performed before and directly after the procedure and at the 6-month follow-up at the angiographic lab of Aachen University blinded to clinical data using a validated quantitative angiographic system (CAAS II System, PieMedical, Maastricht, The Netherlands). ISR was classified, according to the geographic distribution of intimal hyperplasia in reference to the implanted stent, as focal (<10 mm in length) lesion, diffuse intra-stent (>10 mm within the stent), diffuse proliferative (>10 mm extending outside the stent), or occluded ISR12.
Statistical analysis
Categorical data are presented as frequencies and compared with the Pearson chi-square test. Continuous data are presented as mean±SD and compared with unpaired t test or ANOVA as adequate. Follow-up TLR events were analysed with actuarial methods and Kaplan-Meier curves were constructed. Univariate and multivariate regression analysis was performed to identify predictors for recurrent TLR after use of SES for treatment of ISR. Included variables are the reference vessel diameter, lesion length, minimal lumen diameter pre-intervention, minimal lumen diameter post-intervention, diabetes mellitus, lesion location being the left anterior descending artery and use of SES after failed prior IRT. P<0.05 was considered statistically significant.
Results
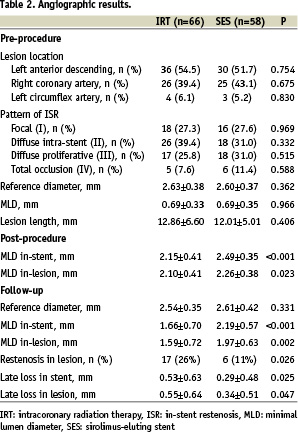
Clinical and angiographic characteristics at baseline were similar in both groups (Tables 1 and 2). There were 16 in-stent restenotic lesions after failed IRT and 56 lesions of first time ISR in the SES group. Intracoronary radiation therapy was used in 64 lesions for first time ISR and in 8 lesions after failed IRT.
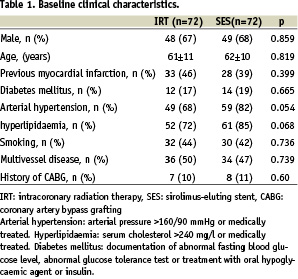
Procedural data
Procedural success was achieved in all cases of the SES group. Predilatation was performed in 51% of lesions. Two lesions were treated with 2 overlapping stents. Mean total stent length was 15.9±5.1 mm. In 3 lesions, high-pressure post-dilatation at 16 atm was used because of a suboptimal result.
Procedural success was also obtained in all patients of the IRT group. Dissection or remaining stenosis required additional bare metal stent placement at the stent margins in nine lesions (12.5%) prior to IRT. The mean radiation doses was 21.1±3.1 Gy at a distance of 2 mm from the radiation source (range 16.0 to 25.3 Gy). Two lesions were treated with dilatation after IRT due to recoil in stent.
Angiographic follow-up
Both groups were well balanced for restenosis lesion length and reference vessel diameter prior to intervention as well as other baseline angiographic characteristics. Quantitative angiographic results for the patients with follow-up angiography are summarised in Table 2. At 6-months, in-stent late loss was 0.29±0.48 vs 0.53±0.63 mm for the SES group compared to the IRT group (p=0.025) and in-lesion late loss was 0.34±0.51 vs 0.55±0.64 mm (p=0.047), respectively. Considering only cases treated for first time ISR, there were 41 lesions in the SES group and 57 lesions in the IRT group with angiographic follow-up. Superior results remained for these SES lesions compared with the IRT lesions regarding late loss and in-lesion MLD at follow-up (0.23±0.41 vs 0.61±0.67 mm, p=0.003, and 2.09±0.54 vs. 1.53±0.64, p<0.001, respectively).
Clinical follow-up
Complete 30-month follow-up was obtained in all patients. There were no acute or subacute stent thrombosis in the SES group. Target lesion revascularisation was required in 7 patients (10%) at 12 month follow-up and in 9 patients (13%) at the 30 month follow-up. Table 3 displays characteristics of lesions with recurrent TLR after SES for ISR. In the IRT group there was one subacute stent thrombosis (1.4%). Recurrent TLR after the IRT procedure was required in 17 patients (24%) during the first 12 months and in 23 patients (32%) within 30 months after IRT for ISR. Thus, the need for recurrent TLR was significantly higher in the IRT group than in the SES group (Table 4). Furthermore, the difference between SES and IRT in the need for TLR increased over time as indicated by an ongoing divergence in TLR free survival curves (Figure 2a).
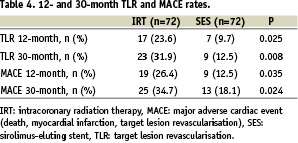
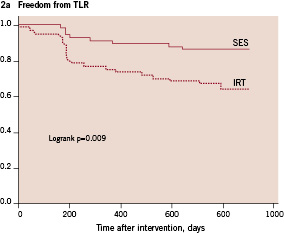
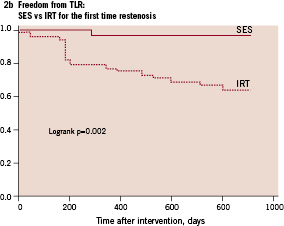
Figure 2. Cumulative target lesion revascularisation free survival of patients with in-stent restenosis treated with either sirolimus-eluting stents (SES) or intracoronary radiation therapy (IRT). Figure 2a demonstrates results of all patients. Figure 2b demonstrates only results of patients treated for first-time in-stent restenosis.
There were 9 patients (13%) in the SES group and 19 patients (26%) in the IRT group who experienced a MACE at the 12-month follow-up. At the 30-month follow-up, there were 13 patients (18%) in the SES group and 25 patients (35%) in the IRT group with a MACE (P=0.024) (Table 4). The MACE rate in the SES group increased between the 12 and 30 month follow-ups due to two non-cardiac deaths in addition to two cases of recurrent TLR.
Treatment of first time in-stent restenosis
In the 56 patients treated with SES for first-time ISR, only two patients (3.5%) underwent TLR within the 12 month follow-up and no additional TLR was necessary in this group until the complete 30 month follow-up. In the 64 patients treated with IRT for first-time ISR, 20 patients (31%) underwent TLR within the 30 month follow-up. Thus, considering only patients treated for first time ISR using either SES or IRT, the difference in TLR as shown by Kaplan Meier survival analysis was even more evident as compared to the total study population (Figure 2b).
Treatment of failed intracoronary radiation therapy
In the 16 patients treated with SES for failed IRT, recurrent TLR at the 12 month follow-up was necessary in five patients and two additional TLR were observed between the 12 and 30 month follow-ups due to late in-stent occlusions presenting as Q-wave and non Q-wave myocardial infarction, respectively. Thus, a total of seven patients (44%) underwent TLR within the 30 month follow-up period. In the 8 patients treated with IRT for failed IRT, 3 patients (38%) underwent TLR within the 30 month follow-up. Considering only patients treated for recurrent ISR after failed IRT, there was no difference in TLR at 30 months between SES and IRT.
SES for first-time in-stent restenosis vs SES for failed intracoronary radiation therapy
Only 2 patients (3.5%) with SES for first-time ISR underwent TLR during the 30 month follow-up. In contrast, in the 16 patients treated with SES for recurrent ISR after failed IRT, 7 patients (44%) required TLR during the 30 month follow-up period. Comparison of patients treated with SES for first-time ISR and patients treated for ISR after IRT failure by Kaplan-Meier survival analysis demonstrated a significant difference in TLR free survival (Figure 3).
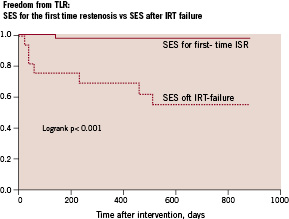
Figure 3. Cumulative target lesion revascularisation free survival of patients treated with sirolimus-eluting stents (SES) for first-time in-stent restenosis (ISR) vs patients treated with sirolimus-eluting stents for recurrent restenosis after failed intracoronary radiation therapy (IRT).
Predictors of recurrent target lesion revascularisation after treatment of in-stent restenosis
Considering patients treated with either SES or IRT for ISR, failed prior IRT, treatment being IRT and pattern of ISR were univariate predictors of TLR. In a multivariate analysis only IRT (P=0.014; OR=2.02, 95%CI=1.15 - 3.56) and failed prior IRT (P=0.016; OR=4.26, 95%CI=1.3 - 13.8) remained independent predictors of recurrent TLR.
Predictors of recurrent target lesion revascularisation after SES for in-stent restenosis
Considering only patients treated with SES for ISR, minimal lumen diameter prior to treatment of ISR and failed prior IRT were predictors for TLR in a univariate analysis. In a multivariate analysis failed prior IRT was the only independent predictor for target lesion revascularisation after SES for in-stent restenosis (P=0.001; OR=134.2, 95%CI=4.10 - 4429.2).
Discussion
This study demonstrates: 1) superiority of SES in comparison with IRT for the treatment of in-stent restenosis over the complete 30-month follow-up period, 2) favourable 30-months clinical results with the use of SES for treatment of first-time in-stent restenosis, 3) a high rate of recurrent and potentially late treatment failure after use of SES for previous IRT failure.
Due to the high antiproliferative properties and effectiveness in the reduction of recurrent restenosis, IRT had emerged as the gold standard for the treatment of ISR. However, repeat TLR rates after IRT for ISR are still between 24-39% considering a 6 to 9 months observation period1,2. Moreover, experimental and clinical data showed delayed lumen loss and attrition of clinical effectiveness over time after radiation therapy for ISR4,5,7,8. A decrease in minimal lumen diameter from 2.49 mm at 6 months to 2.12 mm at the 36 month follow-up has been shown after IRT5. Sianos et al. reported on the outcome of 301 patients treated with intracoronary beta radiation therapy during a mean follow-up period of 3.6±1.2 years8. The TLR rate increased from 12.9% at six months to 28.3% at one year and 50.4% at four years, suggesting a progressive decline of clinical efficacy of IRT over time. The authors concluded that potent antiproliferative therapies designed to reduce TLR require careful late clinical follow-up to ascertain the sustained incremental value.
Sirolimus-eluting stents for ISR
In de novo coronary lesions, sustained clinical safety and efficacy has been demonstrated in randomised, controlled trials up to 3 years after implantation of SES13. Similarly, angiographic and intravascular ultrasound studies performed up to 4 years after implantation of SES for de novo coronary stenoses showed that lumen dimensions remain essentially unchanged between one and four years after stent implantation14. These findings demonstrated that neointimal hyperplasia is effectively suppressed in de novo lesions over a long period without late “catch-up”.
Reports on the use of SES for treatment of ISR evaluated follow-up periods of 6 to 12 months10,11,15-17. These studies have reported in-lesion late loss between 0.16 mm and 0.45 mm and repeat TLR rates between 0% and 19% using SES for first-time ISR. The current study demonstrates a late loss of 0.34±0.51 mm at 6 months and a TLR of 10% at 12 months considering use of SES for both first-time ISR as well as IRT failure. Thus, even considering the complete study population the angiographic and clinical results of this study are well comparable to those of previous studies on the use of SES for first-time ISR during a follow-up period of 12 months. Considering only those patients with treatment for first-time ISR, the 12 month TLR rate was 3.5%. Of note is that there were no additional TLR in this group between 12 and 30 months indicating a remarkable safety and effectiveness of SES for the treatment of first-time ISR during a 30 month follow-up period, even with discontinuation of clopidogrel 6 months after treatment. Thus, there appears to be no attrition in clinical effectiveness over longer follow-up periods if SES implantation is performed for treatment of first-time ISR. Compared to IRT for ISR, the greater effectiveness seems to become even more obvious during longer follow-up periods.
Sirolimus-eluting stents for ISR after failed vascular brachytherapy
Taking into account the enthusiasm on IRT after induction of this treatment modality and the number of procedures performed for the treatment of ISR, recurrent ISR after failed IRT provides a significant clinical problem. This has to be acknowledged in particular as no satisfactory treatment strategy has been established to treat this aggressive restenosis process. Use of drug eluting stents for treatment of lesions with failed IRT has been suggested. However, early results using SES for the treatment of IRT failure were not uniformly positive, even considering limited follow-up periods18,19. In two observational studies with angiographic follow-up limited to 6 and 8.5 months, a rate of recurrent angiographic restenosis of 17 and 40% was reported. Thus, an attenuated efficacy of SES in prevention of neointimal growth in the setting of failed IRT was suggested.
Intracoronary radiation therapy has been shown to decrease neointimal growth, but also delay healing, endothelialisation and subsequent restenosis7,20. Continued healing may be accompanied by excessive neointimal growth. A lack of the endothelial coverage with persistent intimal fibrin deposition and inflammation of the vascular segment treated with IRT have been demonstrated in both animal models and in vivo21. Mechanisms, which may explain the adverse events after SES implantation for failed IRT remain speculative. The appearance of the restenotic tissue with echolucent properties after intracoronary radiation therapy has been reported in intravascular ultrasound studies22. This echolucent material is caused by tissue which is acellular and necrotic and lacks connective tissue elements. According to animal models and clinical studies, late stent thrombosis was reported to be the result of impaired re-endothelisation21,23. As no late total occlusions were observed in the subgroup of first time ISR, it may be suggested that the impaired healing process of previously irradiated tissue, rather than adverse effects of the antiproliferative drug and delayed intimal hyperplasia cause the late in-lesion occlusions and adverse clinical events.
In the present study, the rate of recurrent TLR at the 30 month follow-up was 44% in patients with failed IRT as compared to 3.5% in patients with implantation of SES for first-time ISR. Failed IRT was the only independent predictor for TLR after use of SES for ISR. Recurrent TLR between 12 and 30 months was necessary only after SES use for IRT failure. Noteworthy, these patients with late total occlusions presented clinically as acute coronary events. Both events occurred when the patients were on aspirin therapy only. They were not related to the cessation of dual antiplatelet therapy consisting of aspirin and clopidogrel. Thus, the results of this study indicate that ISR lesions after IRT failure present a challenge for SES. The duration of antiplatelet therapy in the present study was 6 months. A prolonged antiplatelet regimen even beyond 12 months may be required to enhance safety of SES therapy after IRT failure.
Study limitation
This was a non-randomised study including patients from two-centres. The overall patient number is limited. However, the SES group consisted of consecutive patients with in-stent restenosis treated during a predefined inclusion period and according to specific inclusion and exclusion criteria. Both groups were matched according to well known predictors for angiographic and clinical restenosis. Thus, the two groups were well balanced with respect to baseline clinical and angiographic characteristics. The aim of the study was to evaluate the clinical effectiveness of both SES and IRT for ISR beyond the 12 month follow-up period. Thus, repeat angiographic follow-up had not been scheduled after 6 month follow-up and recurrent angiography was only clinically driven.
Conclusions
The safety and efficacy of SES in the treatment of first-time ISR is demonstrated by favourable 30 month clinical follow-up results with TLR rates lower than after IRT. A high rate of recurrent, potentially late restenosis with subsequent need for target lesion revascularisation was observed after use of sirolimus-eluting stents for intracoronary radiation therapy failure.

