Abstract
Heart failure is a major health problem and myocardial infarction (MI) is, at the present time, the main cause. Cell therapy hold great promise for use in tissue regeneration and is increasingly used in an effort to improve outcomes in cardiac disease. Recently it has been shown that adipose tissue, in addition to committed adipogenic, endothelial progenitor cells and pluripotent vascular progenitor cells, contains also multipotent cell types (adipose derived stem cells, ADSCs) that, in cell culture conditions, have extensive proliferative capacity and are able to differentiate into several lineages, mesenchymal or not.
ADSCs, similarly to MSCs, express the CD105, STRO1, CD166, CD117,CD90, CD54, CD44 and CD29 surface markers. Moreover, ADSCs are also capable of secreting a large number of growth factors, including vascular endothelial growth Factor, Granulocyte/Macrophage Colony Stimulating Factor, Stromal Derived Factor-1alpha and Hepatocyte Growing Factor.
Adipose tissue can be harvested in large amounts with minimal morbidity and, owing to the large concentration of stem cells contained in a typical harvest, therapeutic doses of ADSCs can easily be isolated in as little as an hour after liposuction, obviating the need for culture. Several studies conducted in porcine AMI models have shown a significant LV functional improvement, with no report of any potentially fatal arrhythmias.
The APOLLO trial, a prospective, double blind, randomised, placebo controlled, sequential dose-escalation study conducted at the Thoraxcenter, Erasmus MC, will be the ‘First-in-Man’ study that will explore the safety and feasibility of ADSC transplantation in patients with acute MI.
Introduction
Despite recent developments in the treatment of coronary artery disease and the introduction of new devices and effective post PCI therapies, the loss of cardiac tissue and the resulting impairment of left ventricular function after myocardial infarction (MI) remains a main cause of heart failure, defining the need to find an effective treatment to ameliorate this major health problem and its economic consequences. The potential of cell transplantation to repair damaged myocardium is attractive and has been widely studied in both experimental and clinical conditions using various cell types1-6. The quest for the ideal cell is still ongoing, as the attributes of the ideal cell and working mechanism of cell regeneration still remain to be defined. Nonetheless, there appears to be a consensus that, among the cells effective in the treatment of heart disease, cells that are autologous, non-embryonic, do not require culturing to obtain a therapeutic dose, and can be administered during the same procedure, may be logistically easier to use and thus may have wider application in catheterisation laboratories.
The most extensively studied and characterised cells, that have been shown to have some of the above mentioned ideal properties, are mesenchymal stem cells (MSCs). MSCs are multipotent, adult stem cells, that can expand in cell culture and are able to differentiate into multiple cell phenotypes including bone, cartilage, neuronal and skeletal muscle progenitor cells, but also into vascular endothelial cells and cardiomyocytes7-13.
Many previous cell therapy trials in patients with AMI, have been using mononucleated bone marrow derived cells (BMCs)14-17, that consist of a heterogeneous cell population. A small fraction of these unfractioned BMCs are MSCs. Results of these trials showed an improvement of regional wall motion, global ejection fraction and, in some cases, a reduction of infarct size in the treated group14,16. Selected MSCs were evaluated by Chen and colleagues in 200418 in 34 patients demonstrating a significant improvement of regional wall motion, global ejection fraction, reduction of infarct size, but also reduction of left ventricular end diastolic volume (LVEDV) in the treated group, confirming the encouraging results obtained from previous preliminary animal studies19-21. So far, these cells have been harvested from bone marrow, however, limitations of this cell source are: 1) the number of MSCs in bone marrow is rather low; MSCs in fact represent < 0.01% of all nucleated bone marrow cells in healthy volunteers (approximately 1 in 25,000 to 1 in 100,000)22,23; 2) donor site morbidity limits the amount of marrow that can be obtained (generally limited to no more than 40 ml)24,25 and consequently 3) the long time required to obtain therapeutic cell doses by ex vivo cell expansion renders treatment in the acute phase of myocardial infarction impractical.
Recently it has been shown that adipose tissue, in addition to committed adipogenic, endothelial progenitor cells and pluripotent vascular progenitor cells, contains also multipotent cell types26,27. This finding has generated major interest because, in contrast to bone marrow, adipose tissue can be easily and safely harvested in large quantities and with minimal morbidity regardless of the condition of the patient, making it an appealing source for cell therapy.
Cell characteristics
Adipose tissue, differently from most tissues, has a remarkable plasticity during life. Although increases in volume can be partially obtained by increasing the amount of lipids stored in the already existing adipocytes (cellular hypertrophy), large increases in volume are usually associated with an increase in adipocyte count (cellular hyperplasia) accompanied by a concomitant expansion and remodelling of the microvasculature supplying these cells28.
The hyperplastic process of the adipose tissue and supporting vascular bed is mediated by a cell population present within the stromal vascular fraction of adipose tissue generally referred to as adipose derived stem cells (ADSCs). These cells, previously supposed to be mono-potent adipocyte progenitor cells, called pre-adipocytes, have actually been demonstrated to have an impressive developmental plasticity27,29 including the ability to undergo multi-lineage differentiation and self-renewal26,30-32 (Figure 1).
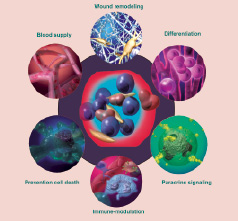
Figure 1. ADSCs actions end effects; ADSCs have an impressive developmental plasticity including the ability to undergo multi-lineage differentiation, to act by paracrine effects and to display an immuno-privileged behaviour.
ADSCs are a cell population with properties that are very similar, though not identical, to those of marrow derived MSCs33-36. These cells have extensive proliferative capacity and are able to differentiate in cell culture conditions into osteogenic, chondrogenic, myogenic lineages7-13,37 and, more recently, into a neurogenic phenotype9,38.
In culture, these cells express surface markers that are similar to those expressed by MSCs (Table 1): both cell types express CD105, STRO1 and CD166 (multi-lineage differentiation markers)39-42, CD117 (stem cell factor receptor), CD90, CD54 (ICAM-1), CD44 and CD29 (beta-1 integrin)13,43,44, whereas both cell types do not express the endothelial markers CD31, CD34 and the haematopoietic marker CD4535,39 (Figure 2).
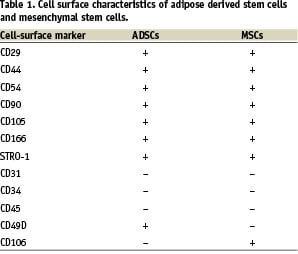
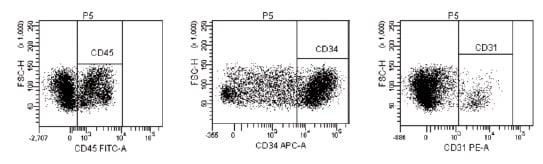
Figure 2. Representative sample of freshly isolated ADRCs. CD45+, CD34bright and CD31bright cells correspond to approximately 38.6, 56.5 and 6.8% of total cells. Cells were analysed using FACSDiva software (Picture from Cytori Therapeutics).
Recent studies showed that freshly isolated human ADSCs from subcutaneous adipose tissue tested positive for the aforementioned markers of haematopoietic and endothelial lineage. Flow cytometric characterisation of human ADSCs in culture revealed that the percentage of cells positive for CD34 expression decreased markedly after 1 week of culture45 suggesting that the adhesion of ADSCs to plastic leads to a selection similar to that seen with MSCs, increasing the proportion of a unique population of cells with immature characteristics46.
One of the most interesting differences in surface makers between MSCs and ADSCs is a reciprocal expression pattern of the very-late-activation antigen 4 or VLA-4 (CD49d/CD29) and its cognate receptor vascular cell- adhesion molecule 1 or VCAM-1 (CD106). ADSCs express VLA-4 but not VCAM-1 in the majority of donors, while MSCs usually express CD106, a component of VCAM-1, and not VLA-435.
The consequences of these properties are not clear yet and needs further investigation, but this observation is fascinating since both these molecules play an important role in haematopoietic stem cell homing and mobilisation from the bone marrow47,48.
MSCs but also ADSCs are known to secrete a large number of angiogenesis-related cytokines45. In ADSCs in standard cultures, high levels of Hepatocyte Growth Factor (HGF), Vascular Endothelial Growth Factor (VEGF), Placental Growth Factor (PGF), Transforming Growth Factor-β (TGF-β), Fibroblast Growth Factor (FGF), Granulocyte/Macrophage Colony Stimulating Factor (GM-CSF), Monocyte Chemotactic Protein-1 (MCP-1) and Stromal Derived Factor-1alpha (SDF-1α) were observed, suggesting an active role of ADSCs in neovascularisation45,49. ADSCs and MSCs both possess the capability to suppress lymphocyte reaction in a dose-dependent and time dependent fashion; such immunosuppression probably occurs via the production of cytokines (including IL-4, IL-10, and TGF-β) responsible for the inhibition of lymphocyte proliferation50,51.
Clonally-derived, multipotent cells from adipose tissue display furthermore an immuno-privileged behaviour33. Less than 1% of ADSCs in fact express the cell surface MHC class-I whereas class- II is almost absent52. Therefore ADSC are poorly recognised by T-lymphocytes, as suggested by the absence of infiltration by myeloid CD3 positive cells. When injected into immuno-competent X-linked muscular dystrophy (mdx) mouse muscles, ADSCs were seen to differentiate into functional muscle cells, integrated by fusing with host cells and were not rejected up to 6 months after transplantation33. This, together with the easy availability and the small amount of tissue needed for their isolation, suggests that, ADSCs might even have potential as immuno-privileged universal donor cells with the capacity to be used not only for autologous but also for allogeneic transplantation.
Harvest and expansion
Subcutaneous adipose tissue can be safely and quickly harvested in large quantities in several regions of the body, using conventional liposuction. A typical harvest out of 100-200 ml of human adipose tissue would yield about 50x106 nucleated cells. During a regular liposuction procedure, it is not uncommon for plastic surgeons to remove in excess of 2,000 mL of fat. The samples are then enzymatically digested, filtered and centrifuged to separate the non-buoyant stromal cells from the buoyant adipocytes46,53,54. The non-buoyant fraction can yield in excess of 1x106 stem cells (from 100 cc of fat tissue), about 45 fold more than a typical harvest of bone marrow aspiration (of 40 ml) which typically contains 2.5x104 MSCs in a mature adult25,55. If necessary, the ADSC containing fraction can be cultured with different media (e.g. DMEM-F12; EGM-2-MV, α-MEM etc.) and supplemented with different percentages of serum and growth factors (e.g. VEGF, Insulin Like Growth Factor, bF Growth Factor etc.) to stimulate differentiation into dedicated lineages and expansion. (Figure 3) Owing to the great concentration of ADSCs normally present in the adipose tissue harvest, it is also possible to instantaneously obtain therapeutic cell doses, with no need for further expansion in culture56. In contrast to BM-derived MSCs, therapeutic doses of ADSCs can easily be isolated in as little as an hour after liposuction45,46. This not only obviates the need for culture, but also avoids cell alterations associated with culturing.
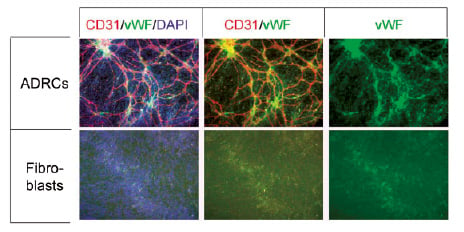
Figure 3. ADRCs form cords in an in vitro tubule formation assay. ADRCs cultured for 1 week form cords in EGM-2. Immunofluorescence was used to detect CD31 (red) and von Willibrand factor (vWF, green). Cell nuclei were detected by DAPI (blue). Adult dermal fibroblasts were used as a control (bottom row).
Differentiation capabilities of ADSCs
Numerous studies have demonstrated that ADSCs, similarly to MSCs, have the capability to undergo differentiation into several cell types along both mesenchymal (fat, bone, cartilage muscle, nerve) and non-mesenchymal lineages (blood vessel)7-13,38,57, demonstrating that adipose tissue actually contains pluripotent cells.
In particular, as far as muscle differentiation is concerned, evidence showed that ADSCs have the ability to differentiate into contractile cells with both striated muscle cells or cardiomyocytes features53,58-62.
In vitro studies of ADSC differentiation
After two weeks of culture in RPMI medium, 20% to 30% of ADSCs increased in size, changed shape and began to beat spontaneously when observed under a phase contrast microscope by three weeks, as observed by Rangappa et al in 200357. Likewise, after three weeks of exposure to rat cardiomyocytes conditioned DMEM medium, ADSCs expressed cardiomyocyte markers including sarcomeric alpha-actin, desmin, and cardiac troponin I, as well as the gap junction protein Connexin-43 (Cx-43) and started to beat spontaneously, as observed by Gaustad et al in 200458.
In 2005, Planat-Benard et al53 obtained similar results by plating fresh ADSCs in a semi-solid culture. After three weeks of culture, ADSCs morphologically developed into ventricle-like, atrial-like and pacemaker-like cells, displaying tight inter-cellular connections (evidenced by Cx-43) and spontaneous, as well as triggered action potentials with beating. Moreover, these differentiated cells expressed the cardiac-specific transcription factors Nkx2.5, GATA-4, and MEF-2C, the structural cardiac proteins β-myosin heavy chain (MHC), myosin light chain-2 ventricular (MLC-2v), myosin light chain-2 atrial (MLC-2a) as well as the atrial natriuretic peptide (ANP), whereas the skeletal marker MyoD and smooth muscle actin were not expressed.
In vivo studies of ADSC differentiation
Several groups have so far reported data about the safety and efficacy of ADSCs to regenerate damaged myocardium in both small (rats) and large animals (pigs).
Delivery of ADSCs by intracoronary infusion to pigs two days after an acute MI through a LAD balloon occlusion (Watanabe et al, 2004) resulted in a 3% absolute increase in LVEF while the untreated pigs showed a 9% decline at 6-month FU (P<0.01)59. Likewise, intracoronary infusion of either ADSCs or MSCs in an acute MI pig model (Valina et al, 2004) resulted in a 11.4% improvement of absolute LVEF after 4 weeks, compared to only 2% improvement in the non-treated group (P<0.003)60.
Strem and colleagues61 in 2005 used intraventricular injections to deliver freshly isolated allogeneic ADSCs from Rosa 26 mice (expressing the beta-galactosidase transgene) into syngeneic recipient mice, following myocardial cryoinjury. Beta-galactosidase positive cells were found in treated mice together with the expression of MHC, Nkx2.5 and with troponin I at 14 days post transplantation, suggesting that ADSCs have the ability to engraft injured myocardium and express specific cardiomyocyte markers in vivo.
Freshly isolated ADSCs given immediately after reperfusion in a porcine AMI model (Alt et al) resulted in a 8% LVEF increase compared to controls at 8-week FU (p=0.023)62.
Interestingly, concordant with the functional improvement, wall thickness and capillary density were both significantly increased in the border infarct areas of the treated group, compared to the control group63.
In a study recently published by Miyahara and colleagues54, ADSCs were transplanted directly onto infarcted murine hearts as a mono-layered cell sheet. After transplantation, the engrafted sheet gradually grew to form a thick stratum that included newly formed vessels, undifferentiated cells and cardiomyocytes. Autologous transplantation of ADSC sheets, resulted in a decreased LVEDP, improved maximum and minimum dP/dt, increased diastolic wall thickness, increased left ventricular fractional shortening and decreased diastolic wall stress, as compared to dermal fibroblast sheets taken as a control.
Human studies of ADSC differentiation
ADSCs have been used successfully in a variety of indications in humans, including the treatment of Crohn’s disease fistulas64,65, osteogenesis imperfecta66 and for breast augmentation and reconstruction after partial mastectomy67. So far, ADSCs have not been used to treat patients with MI or ischaemic heart disease. The APOLLO trial will be the ‘First-in-Man’ study that will explore the safety and feasibility of ADSC transplantation in patients with acute MI. This study is a prospective, double blind, randomised, placebo-controlled, sequential dose-escalation, study that will enrol up to 48 patients with acute MI at the Thoraxcenter, Erasmus MC, Rotterdam.
Eligible patients will be enrolled following primary PCI and will undergo liposuction and isolation of the adipose derived regenerative cells fraction (ADRCs) using the Celution™ system (Figure 4). ADRCs will then be injected within 24 hours from the primary PCI into the culprit coronary artery.

Figure 4. The Celution™ system, a fully automated device for the isolation of adipose derived and adipose regenerative stem cells.
Main safety endpoints will include: major adverse cardiac or cerebral event (MACCE) rates, serious adverse events (SAEs)/adverse events (AEs) rates and a composite clinical endpoint of death, MI, stroke and re-hospitalisation for heart failure. Main feasibility endpoints are: absolute LVEF and changes in LVEF from baseline to six months; MI size; regional wall thickness and thickening in all segments; LV– end systolic volume (LV-ESV); LV– end diastolic volume (LV-EDV); and change in perfusion defect after revascularisation to six months as measured by contrast enhanced MRI, echocardiography and scintigraphy.
Patients will be followed for 36 months and will undergo imaging studies (2D/3D echocardiography, LV angiography, MRI, SPECT), functional evaluation (Left Ventricular Pressure-Volume Relationship), clinical evaluations and laboratory testing at six, 12 and 18 months.
Side effects, adverse events and potential hurdles
One of the biggest issues related to cell therapy is the potential arrhythmogenicity of the transplanted cells. Previous reports conducted using BMCs and skeletal myoblasts showed an increased incidence of arrhythmic events in the treated groups that often led to AICD implantation68.
Though limited safety data are available to date, in vivo preliminary animal studies have not reported an increase in any potentially fatal arrhythmias.
Loop recorder monitoring in pigs treated with ADRCs69 did not show an increase in arrhythmic events throughout the eight week follow-up period (one death in the control and ADRC group within the first week after MI). No sustained arrhythmias (e.g. bradycardia lower than 45/min or tachycardia above 165/min) were recorded in surviving animals. Moreover, at the end of the study period, programmed right ventricular stimulation failed to show an increase in susceptibility for arrhythmias by ADRC treatment. On the contrary, a significantly longer cycle length could be seen in the ADRC group at the beginning of an induced arrhythmia. This finding implies rather a reduction in the overall inducibility of malignant arrhythmias than an increase in susceptibility.
Issues associated with tissue harvest in patients with a recent AMI under high doses of anticoagulants, GP IIb-IIIa inhibitors and Aspirin are a potential concern, owing to the highly developed vascularisation of adipose tissue and the consequent bleeding risk. However, only a relatively small amount of tissue is required to obtain an effective cell dose, and haemostasis at the site of liposuction can easily be achieved by local pressure.
Conclusions
Human adipose tissue is a unique source of multi-potent stem cells that have the ability to differentiate into vascular and cardiomyocytes lineages, secrete several factors with angiogenic and anti-apoptotic effects and demonstrate stem cell-like extensive self-renewal. Moreover, ADSCs/ADRCs, unlike bone marrow-derived MSCs, can be easily and safely obtained in large numbers, without the necessity to culture.
ADRCs have been already used in humans in different clinical settings with excellent results and few side effects. Preclinical studies conducted in animal AMI models showed encouraging results and displayed their in vivo capability to differentiate into cardiac muscle cells as well as to excrete different growth factors with important pro-angiogenic effects that led to a significant improvement of ejection fraction and wall thickness.
The results of the APOLLO trial will provide an understanding of the real safety and efficacy of these cells in AMI patients, giving new insights on their possible future role in the treatment of cardiovascular disease.

