Abstract
Aims: To develop an easily applicable prognostic model that can predict mortality risk in patients undergoing percutaneous coronary intervention (PCI) for non-ST segment elevation acute coronary syndromes (NSTEACS).
Methods and results: A retrospective analysis of 630 consecutive patients undergoing PCI for NSTEACS at our institution between January 1999 and December 2000 (development phase). Multivariate logistic regression analysis to identify independent predictors of mortality. Development of a ‘weighted’ and an ‘unweighted’ risk prediction model, each including the following 8 parameters: age > 65 years, age >75 years, left ventricular systolic function (LVEF) <50%, renal impairment (serum creatinine > 200 mmol/L), multi-vessel (3 vessel) disease, peripheral vascular disease, diabetes mellitus and female gender. Validation of the predictive model on the following 500 patients that underwent PCI over a 20 month period (validation phase). Prognostic models tested for their ability to predict mortality. The derived model was applied to the validation group and the area under receiver operating characteristic curves (ROC) was used to estimate the predictive ability of the prognostic models. The area under the ROC curve on the validation phase was 0.835, signifying a good ability to predict 30 day mortality following PCI.
Conclusion: We have derived a simple easily applicable predictive model based on readily available information that can predict mortality following PCI for NSTEACS.
Introduction
Acute coronary syndrome (ACS) is becoming the most common reason for acute medical admission in the western world.1 Whereas the management of acute ST-segment elevation myocardial infarction (STEMI) has been well defined for some time now, that of non-ST elevation acute coronary syndromes (NSTEACS) has been the subject of debate in recent years. The earlier studies on whether an early invasive strategy is the one of choice in these patients not only demonstrated no benefit, but in some cases even demonstrated an unfavourable early outcome for those subjected to early angiography followed by revascularisation either by coronary artery bypass graft surgery (CABG) or percutaneous coronary intervention (PCI).2,3 More recently, it has emerged that an early invasive strategy with coronary angiography leading to early revascularisation by percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) is the preferable management strategy for those at high risk.4-7 However, there is a need to accurately and individually assess patient risks from such revascularisation procedures in order to decide which revascularisation mode is more appropriate for a given patient, obtain informed consent after discussing with the patient about their individualised risk and plan the peri-operative management of the patient in order to reduce the perceived risks as far as possible. Risk scores for cardiothoracic surgery have been used widely for several years; the need for similar risk prediction models for PCI has been recognised but no such model has been widely accepted or implemented.8-10 This situation might be due to the fact that previous attempts at developing such predictive ‘risk scores’ for PCI have resulted in rather complicated models.11-21 Our aim in this study was to develop an easily applicable prognostic model using readily available data to predict the risk of death in patients undergoing PCI for NSTEACS.
Methods
The study was a retrospective analysis of data collected at the University Hospitals of Coventry and Warwickshire, United Kingdom, and conducted in two phases: the aim of the first or ‘development’ phase of the study was to identify independent predictors of mortality after PCI for ACS and then to develop a risk prediction model; the aim of the second or ‘validation’ phase was to validate the predictive value of the model in a separate cohort of patients.
Development phase
The development phase was a retrospective outcome analysis of 630 sequential patients the results of which have already been published.22 Data was collected in the Cardiology Department of the University Hospitals of Coventry and Warwickshire for every patient undergoing PCI over a 2 year period between January 1999 and December 2000. Primary end-point was one month all cause mortality and secondary end-points were 6 months and 12 months all cause mortality. Patients were eligible for the study if they had PCI for unstable angina pectoris, non-ST elevation myocardial infarction (NSTEMI) or unstable post infarct angina (unstable angina occurring more than 48 hours after the index event and with no evidence of re-infarction). All patients were admitted as acute emergencies. Patients who had PCI for an acute STEMI (‘salvage’ or ‘primary’ PCI) or for stable angina pectoris were excluded from the study. In addition, patients with cardiogenic shock at the time of arrival at the cardiac catheterisation laboratory were also excluded. In total 630 consecutive patients fulfilling the above criteria were included in this phase of the study. The rate of intra-coronary stent usage in this series was 91.3% (575/630). All of those were bare metal stent as at that time drug-eluting stents were not available for use at our institution. All patients received aspirin and heparin prior to and during the procedure and all patients who received stents were given a 2-week course of clopidogrel. Glycoprotein IIb/IIIa inhibitors were given prior to or during PCI in 134/630 (21.3%) of cases. Patients were evaluated in 4 age groups: group A, <55 years (n=118); group B, 55-64 years (n=174); group C, 65-74 years (n=193); and group D, > 75 years (n=145). Risk factors chosen for mortality analysis were age, sex, ethnic group, known hypertension, diabetes mellitus, hypercholesterolaemia, renal impairment (serum creatinine >200 mmol/L), smoking, family history of ischaemic heart disease, previous myocardial infarction, other serious comorbidity, obstructive airways disease, peripheral vascular disease, number of vessels diseased, number of vessels treated by PCI, partial revascularisation (defined as a mismatch between the number of vessels diseased and the number treated), and left ventricular (LV) systolic dysfunction (ejection fraction < 50%).
Calculation of risk score
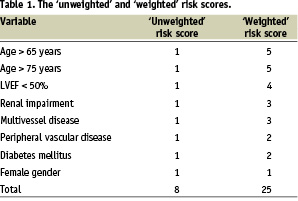
Based on the results of multiple regression analysis and previously published work, an 8 factor risk prediction model was derived (Table 1).11-22 Which factors were included was decided based on a p-value of <0.05 on our multiple regression analysis, as well as those found repeatedly in previous studies to influence outcomes following PCI. This was tested for its ability to predict mortality either as a simple, ‘unweighted’ or a ‘weighted’ risk score by multiple logistic regression analysis followed by the calculation of the area under the receiver operating characteristic (ROC) curve. The ‘weighted’ risk score was calculated as a reflection of the odds ratios of our multiple regression analysis as well as the experience of previous investigators and tailored to make it easily memorable and applicable.
Validation phase
The validation phase was a retrospective application of the risk prediction model derived from the development phase. It included the following 500 consecutive patients undergoing PCI for NSTEACS in the same institution over a 20-month period between January 2001 and August 2002. Selection criteria were the same as in the development period and patients were evaluated in the same 4 age groups: group A, < 55 years (n=110); group B, 55-64 years, (n=141); group C, 65-74 years (n=141); and group D, >75 years (n=108). The rate of use of glycoprotein IIb/IIIa receptor antagonists in the validation cohort was 66.8% (334/500) and the rate of stent usage was similar to that in the ‘development’ phase (460/500 or 92%). During this period, in our institution, the use of drug-eluting stents (Cypher) made its appearance in a very small group of selected patients (37/460 or 8%).
Statistical methods
Statistical analyses were performed using the SPSS Version 10.0 (SPSS Inc) statistical package. Univariate analysis was first performed to evaluate which variables were associated with early (30-day), medium (6-month) and late (1-year) mortality. Those found to be statistically significant (p<0.1, chi square test) were then entered into a multivariate binary logistic regression analysis (forward conditional method) to identify independent predictors of early, medium, and late death. All data were present for the total number of patients in the development phase except for left ventricular function assessed by angiography, which was not done during diagnostic angiography in 226/630 (35.9%) of the cases in the ‘development’ group. Impaired LV systolic function was found to be a significant predictor of 6-month and 1-year mortality when the analysis excluded those with data missing. Therefore, in order to increase the power of the study and avoid excluding 36% of the available data, analyses were performed on all data assuming normal LV function for those in whom LV data were missing. By using this method, the significance of the given variable, if anything, tends to be underestimated. Despite that LV function appeared in most analyses as a significant predictor of mortality following PCI. Analyses were also conducted using other methods (data-imputation and random value assignment) yielding similar results.
Statistical significance for all variables was assumed at p<0.05. Risk was expressed as odds ratio (OR) with 95% confidence intervals (CI). The area under the receiver operating characteristic (ROC) curve was used to evaluate the predictive value of the derived 8 point risk score.
Results
One year mortality for the ‘development’ group was 6.8% (43/630) with an expected increase with age, especially in patients over 75 years. One year mortality was as follows in the various age groups: age <55 years = 0.8% (1/118), age 55-64 years = 1.1% (2/174), age 65-74 years = 6.2% (12/193), and age > 75 years= 19.3% (28/145). Similar mortality trends were observed in this group for 30-day death. The total 30-day mortality was 3.5% (22/630) and increased with advancing age: age < 55 years = 0%, age 55-64 years = 0%, age 65-74 years = 2.1% (4/193), and age > 75 = 12.4% (18/145) (Figure 1).
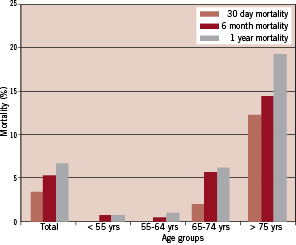
Figure 1. 30-day, 6-month and 1-year mortality in the total development cohort and different age groups (<55 yrs, 55-64 yrs, 65-74 yrs, >75 yrs) of the ‘development’ group.
Univariate analysis in the ‘development’ group revealed age, hypercholesterolaemia, diabetes, impaired LV systolic function, previous myocardial infarction, peripheral vascular disease, renal impairment and partial revascularisation to affect 1-year mortality. Diabetes mellitus (OR 2.7, CI 1.2-6.3), impaired LV function (OR 2.3, CI 1.0-5.4) and peripheral vascular disease (OR 3.1, CI 1.0-9.4) independently predicted death at 1 year. Partial revascularisation predicted death at 6 months (OR 3.6, CI 1.8-9.7) and 1 year (OR 3.1, CI 1.2-7.8). Age > 65 years predicted death at 30 days (OR 18.9, CI 5.5-64.5), 6 months (OR 6.8, CI 3.0-15.0) and 1 year (OR 8.0, CI 3.8-17.1).
Based on these observations and results of previous studies on predictors of adverse outcomes after PCI, we developed a risk prediction model that consisted of 8 parameters: age > 65 years, age > 75 years, impaired LV systolic function (LV ejection fraction {LVEF} <50%, or if LVEF not known history of previous MI or clinical diagnosis of heart failure), renal impairment (serum creatinine >200 mmol/L), multivessel disease (three vessel disease), peripheral vascular disease (including carotid and cerebrovascular disease), diabetes mellitus and female gender. These parameters formed the basis of 2 scoring systems. A simple ‘unweighted’ risk score where all were awarded 1 point with a maximum score of 8, or a ‘weighted’ score system where each parameter was assigned a value between 1 and 5 according to its perceived clinical significance (Table 1).
The two risk scores were first applied in a multivariate regression model on the same cohort (development) and both were shown to be predictors of early, medium, and late mortality (p < 0.001). The area under the ROC curves for both the ‘unweighted’ and the ‘weighted’ prediction models was indicative of a good predictive value (0.738 and 0.818 respectively).
Overall mortality was lower in the ‘validation’ group (n = 500) compared with the ‘development’ group. Thirty day mortality was 2.4% (12/500) and as expected increased with advancing age: age < 55 years = 0%, age 55-64 years = 0.7% (1/141), age 65-74 years = 3.5% (5/141), and age > 75 years = 5.6% (6/108). Similarly, one year mortality in this group was lower at 5.0% (25/500) with the same trends observed in the different age groups: age < 55 years = 0%, age 55-64 years = 1.4% (2/141), age 65-74 years = 7.1% (10/141), and age > 75 years = 12% (13/108). Thirty-day, 6-month and 1-year mortality in the ‘validation’ cohort and the different age groups is summarised in figure 2.
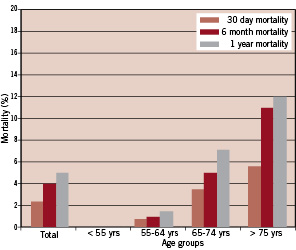
Figure 2. 30-day, 6-month and 1-year mortality in the total cohort and different age groups (<55 years, 55-64 years, 65-74 years, >75 years) of the ‘validation’ group.
Multivariate regression analysis in the ‘validation’ group revealed that both the ‘unweighted’ and ‘weighted’ risk scores were independent predictors of mortality at 30-days, 6-months, and 1-year (p<0.001). The area under the ROC curve for the ‘unweighted’ and ‘weighted’ risk scores as predictive tools for 30-day mortality was 0.831 and 0.799 signifying good predictive values for both (Figures 3 and 4). Similarly, for 1-year death, the area under the ROC curves was 0.774 and 0.788 respectively. Tables 2 and 3 show the incidence of 30-day, 6-month, and 1-year mortality across the different score values in the validation group for the ‘unweighted’ and ‘weighted’ risk scores respectively.
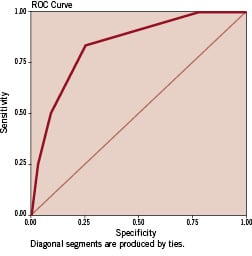
Figure 3. Receiver operating characteristic (ROC) curve for the simple ‘unweighted’ risk score as a predictive tool for 30-day mortality following percutaneous coronary intervention for non-ST elevation acute coronary syndromes (validation phase). The area under the ROC curve was 0.831 signifying a good predictive value.
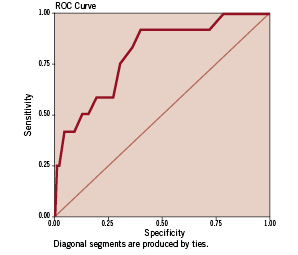
Figure 4. Receiver operating characteristic (ROC) curve for the “weighted” risk score as a predictive tool for 30-day mortality following percutaneous coronary intervention for non-ST elevation acute coronary syndromes (validation phase). The area under the ROC curve was 0.799 signifying a good predictive value.
Discussion
Although elective PCI is associated with a low mortality risk, patients undergoing PCI for acute coronary syndromes exhibit a different pathophysiological milieu which puts them at a higher risk of early or late adverse events. It is important to have a predictive tool to enable discussion with patients and their relatives about potential risks when they are about to consent for PCI following NSTEACS. Rather than presenting patients with the average risk of complication for all patients undergoing PCI derived from large clinical trials, a physician can inform patients which risk stratum they are in and their approximate probability of having a major complication. This ‘individualisation of risk’ would enable proper informed consent. Such a tool can also be useful in identifying which patients should receive new and potentially expensive adjuvant therapies such as glycoprotein IIb/IIIa receptor blockers or an intra-aortic balloon pump. In addition, any attempt at comparing success rates and outcomes following PCI between interventional cardiologists and institutions could be misleading without a standardised system of measuring case mix.
Such a risk score, in order to be generally applicable should be easy to apply. It should contain variables that can be objectively defined and that are readily available for most patients being investigated or treated for the condition.
Published risk prediction models for PCI were mainly developed in high volume single centres in the USA in the early to mid-nineties. These were done before stents and glycoprotein IIb/IIIa inhibitors, which have been shown to have a large impact in risk reduction following PCI, were in wide use. Most of these utilise odds ratio from multiple logistic regression analyses that often yield a complex formula rather than a simple ‘risk score’. A more recent model was again developed in the U.S. at the Mayo Clinic, and although it is relatively simple to apply it does not concentrate on ACS patients and has not become routinely used in European centres.20
Our study has shown that a combination of easily obtainable variables can accurately predict 30-day, 6-month and 1-year mortality in patients with NSTEACS undergoing PCI. This simple risk prediction model can be applied at the bedside in patients being considered for PCI for acute coronary syndromes. We limited this risk score to those undergoing PCI for NSTEACS because this group of patients has a higher as well as a larger range of risks than those undergoing elective PCI for stable angina pectoris.
Our risk score was devised taking into consideration the work of previous investigators as well as the results of our own experience. Variables included were mainly those that were found to be significant in our multiple regression model at the development stage of the study. Clinical variables that did not reach statistical significance in our study but were significant in most of the other research studies dealing with predicting outcomes after PCI were included. Such variables included female gender and renal impairment.
Partial revascularisation, found to be a significant independent predictor of mortality in our study, is a novel variable not previously reported. Partial revascularisation was undertaken in 55.7% (351/630) of the cases in the development phase and 49% (245/500) of the cases in the validation phase. Partial revascularisation usually takes place in the context of ‘culprit’ vessel only being dealt with by angioplasty and stenting. This strategy is acceptable and indeed advisable when the risk of bypass surgery or multi-vessel stenting are perceived to be high during the early stages of acute coronary syndromes. Partial revascularisation is usually sufficient to reduce the immediate risk of adverse events in these patients. However, planned complete revascularisation should ideally take place, where possible, at a later stage in order to avoid medium or long-term adverse cardiac events. In the context of PCI, partial revascularisation is invariably associated with the more widely used variable of multi-vessel disease and, therefore, for the purposes of our risk score this variable was utilised instead.
In the present study we did not include presenting electrocardiographic characteristics or myocardial cell injury markers. These are mainly used during the initial risk stratification process where patients are identified as high risk and likely to benefit from coronary angiography with a view to revascularisation.22-25 No specific anatomical variables were included in our study because previous investigators have demonstrated that coronary and specific procedural characteristics are less important in determining outcomes than simple demographic and anatomical variables.
Study limitations
This study was a retrospective analysis from a single centre database on the outcomes of patients treated with PCI for NSTEACS. These patients were a highly selected group at moderate to high risk, as assessed from their presenting symptoms, electrocardiographic findings, and cardiac troponin T (cTnT) levels. It is likely that the threshold to proceed to coronary angiography and intervention was lower in the younger age groups. In these lower risk groups, the low event rate makes the analysis of co-morbidities more difficult. At the other end of the spectrum, the older the patient the higher the threshold for coronary angiography. In other words, the older age group patients were likely to have more severe and ongoing symptoms than younger patients; ideally we would examine a full consecutive series of patients with NSTEACS from presentation through to discharge, irrespective of angiography or PCI procedures. In the setting of a tertiary referral centre, with patients transferred from a wide geographic area, this would be a formidable task.
The number of patients in this study (n = 1130) is relatively small when compared with previous studies as this is the experience of a single centre over a 44-month period. Restricting the study to a single centre and a relatively short period of time avoids confounding factors and differences in practice between different centres. There was a noticeable reduction in observed mortality of the ‘validation’ group compared with the ‘development’ group. This was despite the mean ‘risk score’ value in the development group being lower than that of the validation group (1.57 v 1.72). The difference in mortality seen between the two cohorts probably reflected the greater use of glycoprotein IIb/IIIa receptor inhibitors in the validation group (66.8%) as compared with the development group (21.3%); the lower rate of partial revascularisation in the validation group (49.0%) as compared with the development group (55.7%) may also have contributed. Age was the strongest predictor of mortality following PCI for NSTEACS but did not significantly differ between the development and validation groups (mean 65.3±0.4 vs 63.8±0.5 years, p = ns).
In conclusion, easily obtainable clinical data can identify a group of NSTEACS patients at higher risk following PCI. Identifying these patients in a more rational way using our simple risk score would enable better consenting processes and comparison of risks with other treatment modalities, thereby enhancing patient management. Additional research is necessary to validate the proposed model prospectively.

