Abstract
Multiple reports describe the high clinical morbidity and mortality associated with contrast-induced nephropathy (CIN). Similarly, reports have described the glomerular filtration rate (GFR) and worsening renal function as predictors of adverse short and long-term outcomes in several large cardiovascular patient populations including acute myocardial infarction, congestive heart failure, coronary artery bypass surgery (CABG), and endovascular aneurysm repair (EVAR). Targeted renal therapy (TRT™) is a novel emerging treatment where high-dose fenoldopam (FEN), a selective renal dopamine-1 receptor agonist and renal arteriolar vasodilator, is infused into both renal arteries via the US FDA-approved 5 Fr bifurcated Benephit PV Catheter Infusion System. TRT™ has been shown to significantly increase the GFR by 25% vs. placebo and IV-FEN (p < 0.001), which may have important clinical implications in CIN prophylaxis and during surgical procedures including CABG. The aim of this report is to review the early clinical experience and pilot trials with TRT™ in several clinical scenarios at high-risk for CIN or worsening renal function including percutaneous peripheral vascular interventions (PPI), percutaneous coronary interventions (PCI), CABG and EVAR.
Introduction
It is estimated that in the U.S. there are 15-18 million patients with peripheral arterial disease, 18-20 million with diabetes (DM), and 50 million patients or 18% of the US population with chronic renal insufficiency1-3. In 1998, the U.S. National Kidney Foundation task force on cardiovascular disease recommended renal insufficiency be considered at the highest risk category for cardiovascular events and contrast induced nephropathy (CIN) be reduced due to an increase in cardiovascular death and mortality even in the patient with mild renal dysfunction4. Similarly, recent data demonstrated renal insufficiency and glomerular filtration rate (GFR) to be strong predictors of adverse short and long-term outcomes after acute myocardial infarction, congestive heart failure (CHF), CABG and endovascular abdominal aortic aneurysm repair (EVAR)5-10. This report describes our early pilot safety and feasibility experience with Targeted Renal Therapy™ (TRT™) and a review of this emerging therapy in the nonsurgical and surgical treatments of cardiovascular disease.
TRT™ is a novel technique for catheter-based delivery of various agents to the kidneys via direct intrarenal artery infusion. Our experience involves the use of TRT™ to deliver therapeutic doses (0.2 - 0.4 mcg/kg/min) of fenoldopam (FEN) (Corlopam) for CIN prophylaxis and potentially to treat other clinical situations associated with renal dysfunction. TRT™ is delivered by the Benephit® PV Infusion System, a 5 Fr system that includes an introducer sheath and a separate bifurcated infusion catheter with low profile, atraumatic tips that are easily positioned into both renal arteries (Figure 1).

Figure 1. Benephit PV Infusion System including the introducer sheath and low profile bifurcated infusion catheter arms.
The system is available in 40 cm, 105 cm, and 140 cm lengths. We have used both the percutaneous femoral and brachial artery approach in both endovascular and surgical procedures for patients at risk for CIN or worsening renal function (Figure 2 A-C).
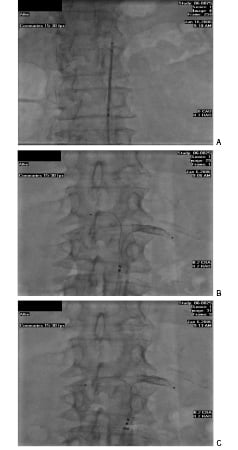
Figures 2 A-C. The Benephit Introducer Sheath positioned in the infrarenal aorta. Note the opaque catheter tips within the sheath (A). The Benephit catheter is advanced and sheath pulled back exposing the preformed catheter infusion arms and opaque tips being first positioned in the left renal artery (note the renal stent) (B). The right catheter arm is “torqued down” into the right renal artery for final TRT™ catheter positioning (C).
The definition of CIN varies but in clinical trials CIN was defined as an increase in serum creatinine (Cr) > 0.5 mg/dl or > 25% of baseline after contrast exposure with peak levels between 3-5 days11-12. The proposed pathogenesis of CIN remains incompletely defined but results in a “vicious cycle” of events that culminates in critical renal cortical and medullary ischaemia, hypoxia and renal cellular necrosis11-12. This cycle includes: a direct cellular cytotoxic effect resulting in increased toxic oxygen-free radicals; intense renal medullary and cortical vasoconstriction mediated by multiple vasoconstrictors including adenosine, vasopressin, and prostaglandin; increase in renal cellular osmolality requiring increased renal cellular oxygen consumption; and a reduction of renal blood flow with endothelial dysfunction and decreased nitric oxide production resulting in renal hypercoagulability, hyperviscosity, acidosis, and worsening ischaemia leading to acute tubular necrosis13-15. Once initiated, there are few therapeutic options to interrupt this cycle.
Fenoldopam trials
Intuitively, renal arteriolar vasodilatation appears protective against CIN because the proposed pathogenesis of CIN is mediated by vasoconstriction followed by renal cellular ischaemia and hypoxia. FEN, a short-acting selective dopamine-1 agonist and renal arteriolar vasodilator, is the only agent shown to increase renal cortical and medullary blood flow16. The initial favourable reports of intravenous (IV) IV-FEN in reducing CIN in percutaneous coronary interventions (PCI) were not reduplicated in the CONTRAST trial17. A limitation of IV-FEN is systemic hypotension as experienced in the CONTRAST trial. Therefore it is theorised that the CONTRAST trial results were secondary to an inability to deliver high intrarenal artery therapeutic doses of FEN to the renal medulla and cortex. TRT™ with high-dose FEN, in concept, has the potential to produce renal vasodilatation and increased arteriolar blood flow without systemic hypotension and therefore has the potential to reduce CIN by negating contrast-induced vasoconstriction.
The early data with TRT™ have been encouraging. The FEN-01 study reported by Teirstein et al validated the feasibility, safety, and efficacy of TRT-FEN delivery and validated several key hypotheses18. Thirty-three patients with renal insufficiency were randomised 2:1 to receive TRT-FEN, then TRT-FEN vs. placebo during PCI. Inulin was used to measure GFR and serum FEN levels were measured. In concept, the TRT-FEN infusion would maximise the favourable renal haemodynamic benefits of FEN while minimising systemic hypotension by “targeted” infusion of FEN.
In the Teirstein report, systemic serum FEN levels were lower with TRT-FEN versus IV-FEN due to the drugs first pass renal metabolism18. Administration of TRT-FEN compared to IV-FEN (0.2 mcg/kg/min) resulted in 30% lower serum levels of FEN18. Additionally, a 45% less reduction in systemic blood pressure with TRT-FEN versus placebo (12+3 mmHg vs. 23+3 mmHg, p < 0.001) was reported18. Importantly, GFR was not significantly altered by IV-FEN but was significantly increased by 25% during TRT-FEN (p < 0.001)18. The GFR increase after TRT-FEN was significantly greater than with IV-FEN (23.6% vs. 4.9%, p < 0.001). Importantly, GFR measured two hours post-procedure remained significantly elevated (25% increase) in TRT-FEN patients while GFR in placebo patients declined 14%18. In a multicentre randomised trial, Tumlin et al found GFR to increase only 4.5% after 30 minutes of IV-FEN versus 19% after 5-10 minutes of TRT-FEN19. This 25% prolonged increase in GFR with TRT™ compared to a 14% decrease with placebo has potential significant clinical implications in preventing CIN and improving outcomes in clinical situations that could benefit from increased renal blood flow therefore GFR.
CIN in PCI
The clinical impact of CIN in PCI was described by McCullough et al identifying significant increases in morbidity and mortality associated with CIN20. CIN is now the third leading cause of hospital-acquired acute renal failure (ARF)20. McCullough reported a 14% incidence of CIN in 1,826 PCI patients with a 7.1% in-hospital mortality in CIN patients not requiring dialysis and 35.7% if requiring dialysis (p < 0.001)20. The in-hospital mortality was 0.7% without CIN. When dialysis was required, the patient had < 30% two-year survival20. Gruberg et al reported a 37% incidence of CIN (7.3% requiring dialysis) in 440 PCI patients with baseline renal insufficiency (Cr > 1.8mg/dl) with three times higher in-hospital mortality (14.9% versus 4.9%) and two times higher 1-year mortality (37.7% vs. 19.4%) in patients with CIN21. Likewise, Levey et al noted a 5.5 fold risk for increased mortality in patients undergoing diagnostic studies requiring contrast exposure who developed CIN4. Clearly the clinical impact of CIN in PCI patients is significant.
Manoharan et al recently reported the assessment of renal flow and flow reserve in humans analysing the renal hyperaemic response to direct intrarenal artery bolus injections of 50 µg/kg dopamine, 30 mg Papaverine, 600 µg isosorbide dinitrate and 0.8 µg/kg FEN. These authors concluded that 50 µg/kg bolus of dopamine elicited the maximal hyperaemic response and suggested the widespread availability and low cost of dopamine as would be reasons to consider dopamine as the most convenient means to achieve increased renal blood flow. The Manoharan report underscores the potential for multiple non-FEN TRT™ strategies that could improve clinical outcomes utilising the direct renal artery access provided by the Benephit Infusion System22. An analysis of TRT™-dopamine is under way.
TRT™ in peripheral arterial disease
The incidence and impact of CIN in percutaneous peripheral interventions remains unknown. Mehran et al and others have identified CIN predictors in PCI including diabetes mellitus (DM), age > 75 years, female gender, contrast volume, Cr clearance (CrCl), Congestive Heart Failure (CHF), hypotension, preprocedure renal insufficiency, the need for Intra Aortic Balloon Pump (IABP), and anaemia and validated a CIN risk score prediction model23. The incidences of these CIN predictors are greater in the peripheral interventions versus PCI population. The individual incidences of DM and preprocedural renal dysfunction in PCI trials are approximately 20%, but these incidences have been reported as high as 50-80% in patients with peripheral arterial disease and critical limb ischemia24. This becomes significant when considering the combination of DM and renal insufficiency was shown by Parfey et al to increase the incidence of CIN during PCI to 50%25. Srondon et al recently reported a 46% rate of renal insufficiency in patients undergoing vascular interventions26.
Additionally, there are several clinical and periprocedural differences during peripheral interventions vs. PCI that increase the risk of CIN. These include: complex, longer case durations with higher contrast use; higher rates of multiple procedures and secondary re-interventions; overall higher haemorrhagic and thrombotic complication rates during vascular interventions; more frequent computerised tomography angiography (CTA) use; and a higher incidence of renal artery stenosis. A typical CLI patient would be a > 80-year-old female weighing 90 pounds, haematocrit of 27.5%, serum Cr of 1.9 mg/dL and a calculated CrCl of < 30 mL/min. The peripheral vascular patient is at a higher risk for developing CIN and therefore strategies to prevent CIN are essential.
Our institute reported early safety and feasibility data in 34 high-risk peripheral vascular interventional patients with TRT™ durations of 1-24 hours (mean=4.5)27. The pre-procedure serum Cr and CrCl were 1.4 - 3.2 mg/dL (mean=1.9) and 15-54 ml/min (mean=34) respectively. There were no device related complications and bilateral TRT™ catheter cannulation was easily accomplished (mean time to cannulation 2.5 minutes). CIN developed in 1/34 (3.0%) patient, which resolved at day 7. The predicted incidence of CIN in this cohort of patients using the validated Mehran score model was 31.5%. The Peripheral Angiographers’ Targeted Renal Infusion for Contrast Induced Avoidance (PATRICIA) trial, a multicentre randomised TRT™ trial has started enrolment in the U.S. to validate efficacy during vascular interventions in high-risk patients for CIN (Figure 3A).
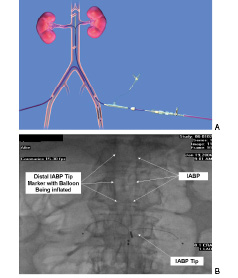
Figures 3. Contralateral TRT access during left leg peripheral intervention with Benephit PV Infusion System in place (A). TRT catheter in place with an IABP during high-risk CABG (B).
The Benephit Renal Infusion Therapy (Be-RITe) Registry, a non-randomised, multicentre U.S. registry, has accumulated data in 366 high-risk patients undergoing coronary and peripheral endovascular and surgical revascularisations. Bilateral renal cannulation occurred in 94% with mean cannulation time of 2.05 minutes with 8.0 cc contrast use. The mean TRT™ duration was 180.1 minutes with a 2.5% incidence of minor hypotension. The mean CrCl was 44.3 ml/min. CIN developed in 9.5% and dialysis in 1.43%. The Mehran CIN score predicted incidences of CIN and dialysis at 29.7% and 3.37% respectively in this patient population. There was 1 minor artery renal dissection treated easily with stenting.28
TRT™ in cardiac surgery
Acute renal failure remains a major morbidity and mortality after cardiac surgery with a reported incidence of 1-2% in low-risk to 30% in high-risk cardiac surgery29-30. Mortality rates are reported of 7-38% without haemodialysis to 60-100% if haemodialysis is required29-30. It is estimated 14% of all cardiac surgical patients have pre-existing renal dysfunction therefore are at increased risk for worsening renal function29-30. Renal failure after cardiac surgery is associated with multiple aetiologies including extracorporeal circulation which has been shown to decrease renal plasma flow, increase renal vascular resistance and decrease GFR by 30% during “on-pump” cases29-31. Interestingly, contrast administration < 48–72 hours before cardiac surgery is an independent preoperative predictor of postoperative renal dysfunction after cardiac surgery but no guidelines exist for perioperative renal protection30-32. The association of CIN during cardiothoracic surgery is unknown, underestimated, and likely a contributing factor to a significant number of patients yearly who develop postprocedural renal insufficiency.
Avoiding renal dysfunction is imperative when considering short and long-term CABG outcomes are associated with even mild degrees of renal insufficiency (Cr=1.5-1.8 mg/dl). In an analysis of 26,506 CABG patients from the Ontario, Canada Cardiac Case Network Database, Lok et al reported overall in-hospital, 30 day, and 1 year mortalities to be associated with renal insufficiency with the 1 year mortality for moderate-severe renal dysfunction of 29.3%, mild renal insufficiency of 11.1%, and 3.8% with no renal insufficiency (p < 0.0001)3. The clinical implications are significant considering that 18% (> 50 million) of the U.S. population has renal insufficiency and 14% of all CABG patients have preoperative renal insufficiency, therefore at risk for perioperative renal failure3,29,30. Hillis et al identified GFR as a powerful, independent predictor of mortality after 2.3 years follow-up in 2,067 consecutive CABG patient in the U.K.9 Cooper et al identified preoperative renal insufficiency to be common and operative mortality rose inversely with declining renal function in 483,914 CABG patients reported to the Society of Thoracic Surgery National Database8. The operative mortality rose from 1.3% for patients with normal renal function to 9.3% for patients with severe renal dysfunction and was less than 9.0 for patients already on dialysis8. Ranucci et al reported a reduction (from 22% to 11%; p=0.28) of renal failure and a significantly lower mortality rate (6.5% vs. 15.7%; p=0.03) using a 24 hour IV-FEN infusion in 108 high-risk CABG patients32. Efficacy could not be confirmed during multivariate analysis due to the small sample size but IV-FEN was an independent protective factor in the low cardiac output subset32.
A safety and feasibility CABG-TRT™-1 pilot trial in 10 high-risk patients has been completed at our institute. The TRT™ catheter was inserted and the FEN infusion started at 0.4 mcg/kg/min in the cath lab immediately pre-op. TRT™ was continued during the CABG and stopped 1 hour post-op. The preprocedure serum Cr and CrCl were 1.5-2.3 mg/dL (mean=1.9) and 21-55 ml/min (mean=39) respectively. There were no device related complications. At 24 and 72 hours postop, 8/10 (80%) CrCl improved, 1/10 CrCl (10%) remained unchanged, and in 1/10 (10%) the CrCl worsened by 25%. The worsened CrCl returned to baseline on day 7. All patients were alive and none required re-hospitalisation or dialysis within 30 day follow-up. TRT™ has been successfully utilised in 10 additional high-risk CABG patients including during IABP and with longer TRT™ infusions of 12-24 hours (Figure 3B). A prospective, randomised, multicenter efficacy trial is being organised to validate TRT™ during CABG.
TRT™ in EVAR
A heightened awareness of renal function and CIN is mandatory during treatment of aortic aneurysmal disease with EVAR now available for the entire aorta and with acceptance of CTA screening protocols. Renal failure after EVAR is also associated with preoperative CrCl and has high post-procedure mortality and morbidity33-35. The incidence of renal dysfunction post-EVAR has been reported from 6-39% and is the third most common experienced morbidity35,36. Interestingly, few reports exist implicating CIN as a prevalent aetiology37-40. Suerwiec et al reported a significant decline in renal function 3 years after EVAR versus open repair implicating repeated CTA scans as the aetiology37. Carpenter et al reported a 20% incidence of pre-op renal insufficiency in 98 EVAR cases with an average volume of intraoperative contrast use of 152 cc (range 35-420) underscoring the potential for intraoperative associated CIN38. Permanent renal dysfunction was reported at 16% in this series. Moore et al recently reported no benefit of N-acetylcysteine in CIN prevention during EVAR in a randomised multicentre trial39. With the rapid adoption of CTA for the diagnosis, treatment planning and follow-up of TAA, AAA and vascular patients, the additional 75 - 125 mL contrast with CTA will mandate strategies to minimise CIN.
A safety and feasibility EVAR-TRT-1 pilot trial in 10 high-risk patients for CIN has also been completed at our institute. The TRT™ catheter was inserted percutaneously via a left brachial approach and a TRT-FEN infusion started in the cath lab immediately pre-op. FEN was infused at 0.4 mcg/kg/min for the duration of the procedure and removed 1-hour post procedure. The pre-op serum Cr and CrCl were 1.6-2.6 mg/dL (mean=2.0) and 18-59 ml/min (mean=30) respectively. Supra-renal fixation devices were not used. There were no device related or EVAR deployment complications. The contrast use ranged from 50-200 ml (mean=155). At 72 hours postop, 7/10 (70%) CrCl improved, 2/10 (20%) remained unchanged, and 1/10 (10%) developed mild CIN, which returned to baseline on day 4. All patients were alive and none required re-hospitalisation or dialysis within 30 day follow-up. A prospective, randomised, multicentre efficacy trial is being organised validate TRT™ use in EVAR.
Therapeutic TRT™ (T-TRT) in non-CIN clinical situations
Interestingly, the proposed pathogenesis of acute renal failure in CIN and sepsis are similar with the final common pathway leading to renal vasoconstriction with the resulting sequelae culminating in acute tubular necrosis40-42. In the U.S. each year, sepsis results in 210,000 deaths, which exceeds the reported deaths due to myocardial infarction40-43. Renal failure occurs in 23-51% of the 700,000 patients yearly who develop sepsis with a mortality rate of 45-70%40-43. Renal failure occurs in 51% of sepsis patients with positive blood cultures40-44. The mortality of sepsis with renal failure is 70% versus 45% with sepsis without renal failure therefore identifying the combination of renal failure and sepsis as a major global healthcare concerns40-43. Landoni et al recently reported a meta-analysis of 16 randomised trials treating 1,290 critically ill ICU patients with IV-FEN44. This analysis concluded that IV-FEN reduced the need for acute kidney injury, the need for renal replacement therapy, in-hospital mortality and shorter ICU stays44. We propose the term therapeutic TRT™ (T-TRT) used in reference to non-CIN related treatment utilising TRT™.
Morelli et al reported a prospective, double blind, placebo-controlled prophylactic low dose (0.09 mcg/kg/min) IV-FEN trial in 300 patients with sepsis45. This study demonstrated the safety of IV-FEN in sepsis and the incidence of protocol defined renal failure, length of ICU stay, and incidence of serum Cr were significantly lower in the IV-FEN group. However the probability of death and the difference in the incidence of severe renal failure and dialysis demonstrated only a favourable trend not reaching statistical significance45.
In early sepsis, vasoconstriction with intact tubular function predominates, therefore it could be theorised that early T-TRT with high-dose therapeutic FEN could be an effective early treatment strategy45. This early interventional T-TRT strategy certainly appears reasonable when considering there are no proven therapeutic or interventional strategies to prevent dialysis due to CIN, sepsis, or a combination of both as likely occurred in our CLI patient.
We recently reported a high-risk CLI patient with a CrCl of < 30 ml/min who underwent vascular intervention with TRT™ initially without the development of CIN46. Four days later, the patient developed sepsis and bacteraemia, followed by oliguric acute renal failure requiring 3 days of urgent dialysis. T-TRT-FEN was started simultaneously with dialysis after three dialysis treatments. T-TRT was continued for 24 hours with an immediate return of urine output and improved renal function allowing “rescue” from dialysis after 4 treatments. The CrCl returned to baseline within 2 weeks. We subsequently treated a post-CABG patient with normal preoperative renal function who developed acute renal failure due to an aortic rupture, hypovolemic shock, and massive blood transfusions. A 24 hour post-op T-TRT-FEN infusion facilitated renal recovery without dialysis. The mechanisms of action of T-TRT in these non-CIN clinical situations could be theorised as immediate reversal of the vasoconstriction cycle of acute tubular necrosis secondary to the renal arteriolar vasodilator effect of TRT-FEN.
Cardiorenal syndrome and refractory CHF are additional clinical situations in which renal function and GFR are associated with outcomes6,7. It is theorised that T-TRT could facilitate CHF treatment especially during episodes of acute decompensation that often times require ICU admission, ventilator requirement, and urgent dialysis. Several anecdotal refractory CHF cases have been successfully treated with T-TRT utilising FEN or nesiritide (Natrecor) (personal communications). Similarly, many patients awaiting cardiac transplantations ultimately require dialysis before transplantation becomes available therefore adversely affecting overall outcomes. Theoretically CHF and transplantation patients could benefit from non-CIN T-TRT strategies including infusions of nesiritide, prostaglandins or future agents.
Conclusion
The unique ability of TRT-FEN to increase GFR by 25% is attractive when considering multiple recent reports now associate a declining GFR to worse overall cardiovascular outcomes after acute myocardial infarctions, CABG, EVAR and in CHF. TRT™ is a safe, feasible and promising approach for CIN prophylaxis and avoiding worsening renal function during the treatment of high-risk endovascular and surgical patients. Additionally, the Benephit Infusion System may provide a vehicle for T-TRT using a spectrum of cardiovascular treatments in multiple non-CIN clinical situations with the potential to improve clinical outcomes. Prospective, randomised, multicentre validation trials are warranted and under way to confirm the initial promising early experiences of this novel therapy.
Acknowledgements
The authors wish to thank Ms. Kelly Tilbe for her assistance with manuscript preparation.

