Abstract
Aims: Fractional flow reserve measurement is based upon achieving maximum hyperemia. A 40 µg intracoronary (IC) adenosine bolus sometimes seems insufficient, and we therefore sought to assess the possible role of 100-150 µg boli in routine.
Methods and results: 108 intermediate (49±16%) stenoses were consecutively studied with 6F catheters. A history of myocardial infarction in the territory of the explored artery or myocardial hypertrophy were the exclusion criteria. Mean FFR was 0.82±0.12 with a 40 µg adenosine bolus and decreased to 0.80±0.12 and 0.80±13 respectively with 100µg and 150 µg boli (P<0.001 vs 40µg in both cases; 100 vs 150 µg, NS). The 40 µg bolus failed to diagnose 8 out of 30 (27%) significant stenoses (i.e., final FFR <0.75). The large boli led to 12 (11%) transient asymptomatic and spontaneously resolving AV blocks without other side-effects.
Conclusion: FFR underestimated a quarter of intermediate stenoses with the currently used 40µg IC adenosine bolus. A large bolus up to 150 µg appears to be accurate and safe for routine FFR measurement.
Background
A Fractional Flow Reserve (FFR) value below the 0.75 threshold is a means of assessing the significance of a coronary stenosis during coronarography1,2. An intracoronary (IC) adenosine bolus of between 20 and 40 µg is widely employed3, even though certain studies have suggested that maximum hyperemia - which is essential4 - is not always achieved with such doses5,6. We therefore sought to assess the possible role of a larger (100 - 150 µg) IC adenosine bolus in routine FFR measurement.
Methods
All suspect coronary lesions (stenosis between 40% and 70% on quantitative angiograms, AdvantX, GEMS ) without prior non-invasive test were subjected to FFR measurement. The exclusion criteria were: acute myocardial infarction (MI), history of MI in the territory of the explored artery, or myocardial hypertrophy. After IC injection of 0.2 mg isosorbide dinitrate, coronary pressure measurements were performed (0.014 inch PressureWire, Radi Medical) as previously described3 using a 6F guiding catheter without side-hole. The entire procedure was performed meticulously, with special care being taken to preserve precise pressure balance and avoid spillover during the bolus. Every 2 minutes, FFR was measured successively with 40 µg, 100 µg and 150 µg boli, irrespective of the artery. P-wave blocks and any side-effects reported by the patient were systematically noted for each bolus. For each adenosine bolus, the measurement in seconds of the sustained minimal FFR value was defined as the hyperaemic plateau duration.
Statistics
Results are presented as percentages or mean±SD. Inter-group comparison was by matched t or Wilcoxon test. The statistical significance threshold was set at P<0.05.
Results
Between June 2003 and June 2004, pressure-derived fractional flow reserve was measured in 108 coronary stenoses (% stenosis = 49±16%) in 82 consecutive patients. Patient characteristics are shown in Table 1.
Mean FFR was 0.82±0.12 with a 40 µg adenosine bolus and decreased to 0.80±0.12 and 0.80±13 respectively with 100 µg and 150 µg boli (P<0.001 vs 40µg in both cases; 100 vs 150 µg, NS) (see Figure 1). The hyperaemic plateau increased from 12±2 seconds with 40 µg bolus to respectively 16±3 and 20±4 seconds with 100 µg and 150 µg boli (all, P<0.0001) (see Figure 2).
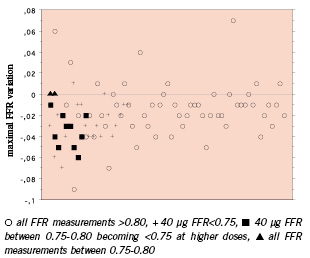
Figure 1. Representation of the maximum variation in FFR measurements between 40 µg adenosine bolus and higher boli (100 or 150 µg) for each intermediate stenosis studied. Stenoses are classified according to the initial and the minimum FFR obtained.
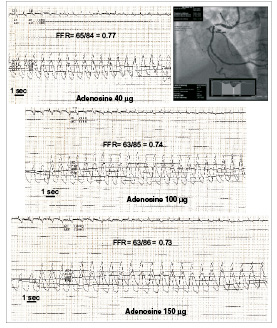
Figure 2. Representative example of FFR measurements with incremental boli of adenosine for an intermediate proximal right coronary artery stenosis (45% stenosis, minimum lumen diameter 1.6 mm). Note that the duration of hyperemia increases with the dose.
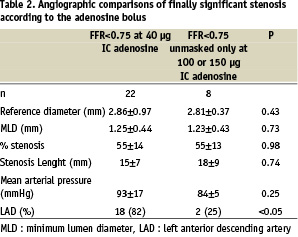
The 40 µg bolus resulted in an FFR of <0.75 for 22 of the stenoses; finally, however, 30 FFR measurements were <0.75, 8 of these only showing up with ≥100µg bolus (i.e., 26.7% of significant stenoses). Stenoses with an FFR value <0.75 with a large bolus of adenosine ranged from 0.75 to 0.78 with a 40µg. No angiographic predictor could be identified except the LAD location (see Table 2). In one patient (3.5%) the FFR was larger than 0.80 with a bolus of 40µg but decreased to less than 0.75 with a larger bolus of adenosine.
Auriculo-ventricular block, never longer than 3 seconds and always resolving spontaneously, occurred 12 times (11%), 6 of these during RCA exploration, and only once with a 40 µg bolus. No other side-effects were observed.
Discussion
Maximum hyperemia and adenosine
Maximum hyperemia is the keystone for FFR measurements3. Short coronary ischemia is the most reliable experimental procedure7,8; in humans a variety of molecules have been used to achieve maximum hyperemia3,8,9, but their effectiveness in any given patient cannot be demonstrated. Thus, between 8% and 16% of patients are liable to achieve only sub-maximal hyperemia with a ≤20µg adenosine bolus3-6, entailing a risk of overestimating the FFR. The fact that there was no significant difference between FFR on 100µg and on 150µg suggests that maximum hyperemia had been achieved. These findings match animal data: in anesthetised dogs, a 100µg IC adenosine bolus achieves hyperemia comparable to 30 sec ischemia, which a 40 µg bolus fails to8. The absence of maximum hyperemia in a non-negligible number of patients with lower doses of adenosine has not been clearly explained. Modeling has shown an increase in microvascular resistance to overestimate FFR10, and Meuwissen et al. found a quarter of patients with 40%-70% stenosis to show significant variability in microvascular resistance under hyperemia induced by ≤20 µg adenosine11. Our high dose, at ≥100 µg adenosine, may be presumed to have lowered this resistance (or at least contained its variability) - which even 40 µg failed to - making the endocoronary pressure measurement more reliable.
Accurate measurement of FFR
High IC doses of adenosine up to 210 µg unmasked 9/17 (53%) of significant coronary stenoses undetected with an initial 15µg bolus6; 8 of these 9 had FFR between 0.76 and 0.79 at 15µg. Another study showed that a 42-48µg versus 12-18 µg bolus unmasked 9/40 initially underestimated stenoses (22.5%)12. We found a quarter of significant stenoses to go undetected with IC adenosine boli ≥100 µg. Our incremental doses started off at 40µg, which could be considered to be high but even so 8 finally significant stenoses had an initial FFR between 0.75 and 0.80; commencing from 15µg or 20µg, the pattern would probably have been more pronounced.
Mean blood pressure tended to be lower (84±5 mmHg vs 93±17 mmHg, NS) in patients with FFR<0.75 overestimated by 40µg IC adenosine. This finding should be seen in the context of modeling showing that a given intermediate stenosis can have a FFR overestimatation (up to+0.08) when blood pressure is reduced from 130 to 70 mmHg10.
Tolerance for high dose IC adenosine
Similar to other investigators6, we found only transient asymptomatic AV blockage that resolved spontaneously, which suggests that a 150µg adenosine bolus is acceptable for routine use. Due caution should be given to this concept, given the pain3 and discomfort5 associated with the continuous intravenous adenosine administration recommended for maximal hyperemia4.
Conclusion
We show a 150 µg IC adenosine bolus to be safe and improves routine detection of functionally significant coronary stenoses, as compared to the classic 40 µg dose. A 40 µg bolus underestimates a quarter of stenoses finally shown to have FFR less than 0.75.

