Abstract
Aims: Recent evidence suggest that coronary bifurcation lesions might be treated by DES using “the mini-crush technique” with low rate of MACE and restenosis both at main and side branches. However, the treatment of a coronary trifurcation lesion is more problematic. Here we assess the feasibility of the “mini-crush technique” for treating trifurcation lesions.
Methods and results: We report on the treatment of trifurcation lesions using DES in all branches by the mini-crush technique in five consecutive patients (65±11.5 years) from December 2004 till March 2006. Independently, from the anatomical type of trifurcation, the mini-crush procedure was performed in all cases. After predilatation of all branches, positioning of stents in both side branches at a distance of 1-2 mm proximally to the carina of the trifurcation was performed. Side-branch stents were then deployed sequentially and crushed at the same time by a balloon positioned in the main branch. Afterwards, the main branch stent was advanced to cover the ostium of both side branches and deployed. The jailed wire technique was employed in all cases, and if possible in both branches. Final triple kissing balloon was employed in all cases.
The “mini-crush technique” was performed safely in all the five patients obtaining an excellent angiographic result at 8.0±1.0 months follow-up angiography.
Conclusions: The “mini-crush technique” with DES can be safely performed giving complete coverage of the ostium of side branches and optimising side branch access.
Introduction
Treatment of bifurcation lesions remains a technical challenge for the interventionalist and is hampered, as well, by an increased rate of restenosis when compared to non-bifurcation lesions1-3. Trifurcation lesions are even more technically challenging, with the potential for both early procedural complications (stent distortion, plaque shift, dissection, side-branch trapping) and late in-stent restenosis4. Part of the complexity in treating these lesions is due to the different anatomic patterns of the stenosis, given the possible involvement of the proximal and the distal part of the main branch and/or side branches, and the close proximity of side branch origin5, besides the angle of side branch origin and the size of both main and side branches.
Some anecdotal cases of trifurcation lesions have been reported in the past demonstrating the importance of triple kissing balloon inflation at the end of the procedure, and the need for DES implantation to achieve an optimal angiographic and clinical outcome6-8. Recent evidence suggests that coronary bifurcation lesions might be treated by drug eluting stent using the “mini-crush technique” with low rate of MACE and restenosis - both at main and side branches9. We thus sought to evaluate the feasibility of this modification of the crush technique by implantation of sirolimus-eluting stents (SES) (Cypher, Cordis/Johnson & Johnson, Warren, New Jersey, USA) or paclitaxel eluting stents (PES) (Taxus, Boston Scientific, Natick, MA, USA) in a consecutive group of patients with a trifurcation lesion.
Materials and methods
Study population
This report includes five consecutive patients with clinically significant de novo trifurcation lesions, who refused coronary artery by-pass surgery and gave informed written consent for a percutaneous approach between December 2004 till March 2006. A trifurcation lesion was defined as diameter stenosis >50% involving the main parent vessel, with or without involvement of side branch ostium, or the origin of the side branch as for bifurcation lesions10. Trifurcation lesions were classified according to the Medina classification previously described for bifurcation lesion10 and adapted for trifurcation lesions (Figure 1).
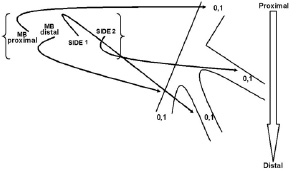
Figure 1. Medina classification adapted for trifurcation lesion.
Angioplasty procedure
All patients were pretreated with aspirin 325 mg and either ticlopidine or clopidogrel. A 300-mg loading dose of clopidogrel before the index procedure was administered if patients were not pretreated. During the procedure patients received intravenous, unfractionated heparin (10 IU/Kg) to maintain activated clotting time between 250 to 300 s. None of the patients underwent the administration of glycoprotein IIb/IIIa inhibitors. After the procedure, all patients were on maintenance aspirin therapy (75 mg daily) and clopidogrel (75 mg daily), continued for at least nine months after DES implantation. Independently from the anatomical type of trifurcation, the “mini-crush technique” which has been previously described for bifurcation lesion9 was performed and adapted in all cases. Briefly, predilatation of all branches was performed sequentially with kissing balloon between the main branch and the distal branch, firstly and between the main branch and the proximal branch, secondly. This was followed by positioning of stents in both side branches (the proximal marker of the side branch stents were situated in the main branch at a distance of 1-2 mm proximally to the carina of the bifurcation). Side-branch stents were then deployed sequentially and crushed at the same time by at high pressure balloon inflation positioned in the main branch. Afterwards, the main branch stent was advanced to cover the ostium of both side branches and deployed. Jailed wire technique was employed in all cases and, if possible, in both branches. This is usually performed to facilitate the stent struts recrossing more closely to the carina of the bifurcation as this choice may influences stent deformation11. Final triple kissing balloon was employed at high pressure, in all cases using a 7 Fr catheter in three of them and an 8 Fr catheter in the remaining two using monorail semi-compliant balloons. Main and side branch balloon sizes were chosen according to vessels size without down-sizing them. As previously described12, intravascular ultrasound (IVUS) was performed in three cases to demonstrate stent expansion in the parent vessel, patency of side branches and full coverage of the bifurcation segment. The IVUS was then repeated in the same patients at follow-up.
All patients were monitored for any post-procedure events of chest pain, heart failure, bleeding, or any ischaemic events. CK-MB and troponin I were measured at six to eight, and 12 to 24 hours post-procedure.
Clinical definition and follow-up
Clinical follow-up was performed by office visits at one month and at eight months. Angiographic follow-up was scheduled between six and nine months after the procedure, unless clinically indicated earlier.
MACE were defined as cardiac death, acute myocardial infarction, and target vessel revascularisation (TVR), either percutaneous or surgical. TLR was defined as a repeat revascularisation with a stenosis >50% within the stent or in the 5-mm distal or proximal segments adjacent to the stent; TVR was defined as repeat revascularisation within the treated vessel. Target trifurcation revascularisation (TTR) was defined as a repeat revascularisation with a stenosis >50% within 5 mm proximal or distal to the carina of trifurcation, both onto the main branch and/or side branches (Figure 2).
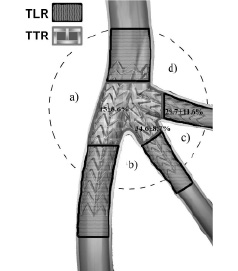
Figure 2. A schematic diagram of the in-stent segmental approach to quantitative coronary analysis (QCA) with the mini-crush stenting. TLR=target lesion revascularisation; TTR=target trifurcation revascularisation; a=angle of main branch deviation; b=angle of first side branch deviation; c=first angle of trifurcation; d=second angle of trifurcation.
Stent thrombosis was defined as an acute coronary syndrome with angiographic documentation of either vessel occlusion or thrombus within or adjacent to a previously successfully stented vessel or, in the absence of angiographic confirmation, either AMI in the distribution of the treated vessel or death not clearly attributable to other causes13. Stent thrombosis were categorised according to the timing of the event into: intraprocedural, subacute thrombosis (from the end of the procedure to 30 days), and late stent thrombosis (>30 days).
Quantitative coronary angiographic analysis (QCA)
Coronary angiograms obtained at baseline, at completion of the stenting procedure, and at 8.0±1.0 months were analysed using a computer-based algorithm developed by CardiOp-B system (Paeion Medical LTD, Rosh Ha’ayin, Israel). The projection that best showed the stenosis in both main branch and side branch in its tightest view was used for all angiograms. One experienced technician who was blinded to patients’ identities, outcomes and sequence of the film, performed quantitative analyses of all angiographic data. Minimal lumen diameter and the nearest normal reference diameter were measured in millimetres using the catheter as scaling factor. Percent stenosis was calculated as 100 (1- minimal lumen diameter/normal reference diameter). Significant binary angiographic restenosis was defined as >50% diameter stenosis of the target lesion. Late lumen loss was defined as the difference in minimal luminal diameter at completion of the stent procedure and during follow-up. Quantitative angiographic measurements of the target lesion were made at the “in-stent” zone (only the stented segment) and at the “in-segment” zone (stented segment and margins 5 mm proximal and distal to the stent). Angiographic success was defined as a final residual stenosis <30% with TIMI flow grade 3 in either the main branch or the side branch14. Procedural success was defined as the achievement of angiographic success without in-hospital MACE. In patients who had restenosis, the pattern of restenosis was evaluated according to the classification of Mehran et al15. For each patient, baseline, post-procedure and follow-up coronary angiograms were analysed.
In none of the patients the angiogram was unreadable or technically deficient for the analysis.
Results
Baseline and procedural characteristics
Baseline clinical characteristics and procedural details for the five patients are shown in Table 1 and 2.
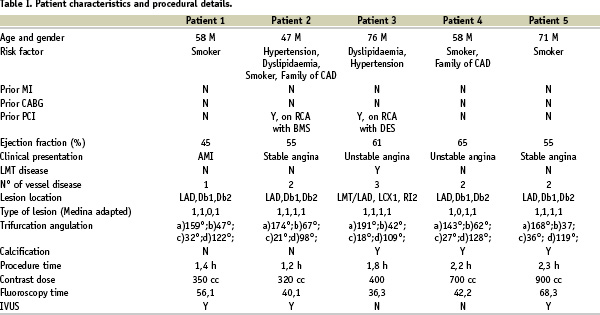
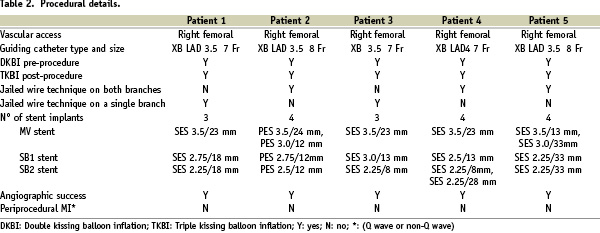
It is interesting to note that none of the patients had diabetes, and that left anterior descending/diagonal branch (LAD/DB1/DB2) lesion location, as well as type 1,1,1,1 trifurcation was present in the great majority of cases (80% and 60%, respectively), Table 1.
Successful delivery of stent to main and side branches was accomplished adequately in all cases (Figure 3), and also confirmed by IVUS in patients No 1, 2 and 5 (Figure 4).
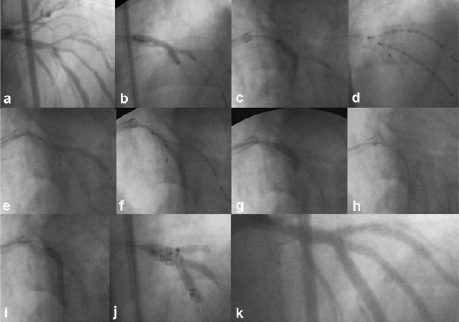
Figure 3. Description of the mini-crush technique in patient number 5. a) Antero-posterior cranial shows critical stenosis of a LAD trifurcation involving first and second diagonal branches; b-c) Kissing balloon was performed between LAD and D2 firstly, and between LAD and D1 afterwards; d) Two stents are advanced into D1 and D2 branches with the proximal stent segment protruding into the LAD for 1-2 mm; e-f) Diagonal branches stents are deployed; g) Crushing of the protruding segments of both diagonal branches stents is performed by balloon inflation on LAD; h) A new stent is advanced into LAD to cover the ostium of both diagonal branches; i) The stent on LAD is deployed without removing both diagonals guidewires (jailed wire); j) First and second diagonal branches were rewired through the LAD stent struts and triple kissing balloon is performed; k) Final result.
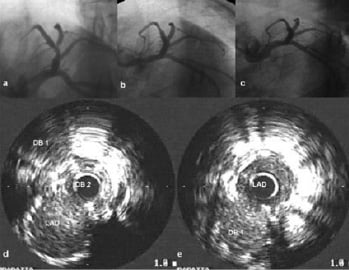
Figure 4. Case No: 1. a) Baseline angiogram; b) Post procedure angiogram; c) 8-month follow-up angiogram; d) IVUS pullback post-procedure in D2; e) IVUS pullback post-procedure in LAD. Please note the complete apposition of both stents and the absence of restenosis at 8-month follow-up.
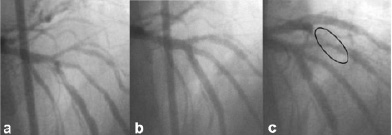
Figure 5. Case number 5. a) Baseline angiography; b) final results after mini-crush technique at LAD-D1-D2 trifurcation; c) 8-month angiographic follow-up showing TLR, but not TTR.
During and after the procedure, occlusion of the side branch did not occur in any case and significant stenosis did not occur in any case. An additional stent in the main branch was required in patient No 2 to seal significant dissections at edges of the study stent, and in patients No 4 and 5 to cover the full lesion length in the second side branch and main branch, respectively.
Angiographic QCA results for both the main and side branches at baseline and after procedure are summarised in Table 3.
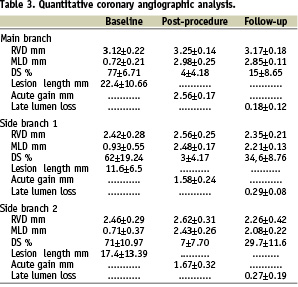
Significant binary restenosis with a focal pattern occurred in 1 out of 10 (10%) side branches.
Clinical in-hospital/30-day results and angiographic follow-up at 8-month was available in all five patients. No patients had in-hospital events, nor at the 30 day follow-up. Only one patient (No 5) presented a major adverse cardiac event at the seven month follow-up angiography which was a target lesion revascularisation due to a first side branch distal stent edge restenosis far from the trifurcation carina. This lesion was retreated because IVUS examination showed at the site of maximal restenosis a minimal luminal area of 1.9 mm2; this was significantly reduced as compared to proximal and distal segments (4.6 and 4.3 mm2 respectively). No differences in baseline lesion characteristics was observed between lesion with restenosis and lesions without restenosis. Indeed, it is interesting to note that no other MACE was reported, and none of the patients showed a target trifurcation revascularisation.
Discussion
Whenever the three stents approach as intention-to-treat in a trifurcation lesion appears needed, the “mini-crush technique” successfully applied in the past for bifurcation lesions, can be safely performed using three DES stents with an high procedural success rate in a consecutive and highly complex patients subset such as those with a trifurcation lesion in a main coronary artery.
Evolution of techniques during recent years, has allowed the interventional cardiologist to approach increasingly complex lesion subsets such as those of a trifurcation lesion by drug eluting stents. The decision to implant three stents from the beginning of the procedure mainly depends on the extension of myocardium at risk supplied by the side branches. It is important to assess the size of the side branches and the extension of the disease into its proximal segment. On the other hand, in very tortuous, angled and calcified lesions it is probably better to accept some amount of residual stenosis, a price frequently paid in cases of provisional stenting, rather than to complete a risky procedure with an optimal angiographic result at the level of the side branch16. Indeed, the recently published randomised SES bifurcation studies emphasise the persistent limitations related to routine stenting of the side branch17-19.
Whenever the complexity of trifurcation lesion suggest the usage of multiple stents as intention to treat, the suggestion of a simple strategy may be difficult to accept at first. In addition, the technical challenges increase even further when the two side branches at the origin of the trifurcation are in close proximity to each other. Indeed, in this situation, a triple kissing balloon inflation seems to be mandatory to achieve an optimal angiographic and clinical outcome, as well as not down-sizing the balloon, which, at least as shown in our study, when the main branch is significantly larger than side branches6-8.
Regarding the technique employed, one obvious concern was that a trapping might occur due to the passage of the stent in the main vessel after the balloon crushing of the side branches; in our experience however, this did not occur in any case. It is also interesting to note that recrossing the stent struts and performing a final triple kissing balloon was possible in all cases similarly to that obtained when treating a bifurcation lesion9.
From the theoretical point of view, separate stent inflations rather that simultaneous stent inflation (as in the standard crush) may significantly reduce the complexity to accommodate three bulky stents at the same time within the trifurcation lesion, as well as favouring more homogeneous and fully stent coverage20-22. In other words ,with this “balloon crushing” there is less risk that the main-vessel stent will be deformed and potentially unapposed to the vessel wall, and thus predisposed to restenosis or stent thrombosis23,24, as previously shown in a bifurcation series of patients9. Indeed, in our study, IVUS imaging of the trifurcation site both at the procedure and at follow-up in patients numbers 1, 2 and 5 demonstrated excellent stent vessel wall apposition and no restenosis at the trifurcation site. Furthermore, this approach may prove to be very helpful, especially in case of an emergency situation where fixing every branch before the final stent implantation in the main vessel may reduce the rate of complications. Eight-month angiographic restenosis provides evidence that this approach pays off, giving a mean in-segment restenosis rate as low as 15+8,6% in the main vessel and 34,6±8,7% and 29,7±11,6% for both first and second side branch respectively (Figure 2). Interestingly, the only patient who showed restenosis, had a side branch stent edge restenosis far from the trifurcation carina. Indeed, none of the patients showed a target trifurcation revascularisation.
Finally, following this mini-crush approach, the limited length of the five layers and the optimised expansion of the crushed side branch stents may have created a predisposition to reduced acute and subacute thrombosis as observed in our cases. Indeed, among the procedure-related factors, stent malapposition and/or under-expansion, stent length, persistent slow coronary blood flow, placement of multiple stents, positive remodelling, dissections and geographic miss – especially seen in the complex setting – appears to be most important for the development of in-stent thrombosis. Large scale, non-corporate-sponsored registries and clinical trials are needed to reliably assess the “true” risk of stent thrombosis with multiple DES in coronary trifurcation lesions.

