
Innovators Day at PCR London Valves 2017 brought together over 300 key stakeholders in the future of transcatheter valve therapies for a day of discussion, networking and reflection. Venture capitalists, economists, industry leaders, scientists, engineers, clinical trialists and clinicians all featured in a programme of teaching, small group discussion and debate to provide perspectives on unresolved issues in the field and predict developments by 2025. A key feature of the innovative programme was a one-hour round table workshop in which participants contributed to small multidisciplinary group discussions facilitated by key opinion leaders (Figure 1) addressing the immediate future of percutaneous valve intervention. Collation and restitution of these discussions determined the content of a final panel discussion with full audience interaction, the conclusions of which are presented herein.

Figure 1. PCR London Valves Innovators Day 2017 – the round table workshop in action.
The aortic valve (Table 1)
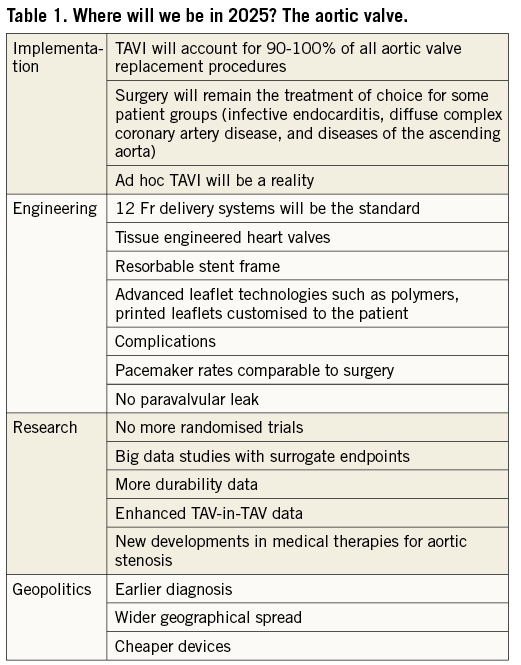
With over 300,000 patients treated to date, indications for transcatheter aortic valve implantation (TAVI) continue to widen. However, despite the outstanding technical achievements and clinical results to date, resistance from surgeons and the lack of randomised long-term data to provide reassurance concerning valve durability have potentially impeded wider adoption. Key issues identified early in the evolution of the procedure – use of the native aortic valve tissue as an anchor and the importance of collapsing the valve for percutaneous delivery before expansion without leaflet damage – still underpin contemporary valve design.
TAVI is now extensively used for patients at high and intermediate risk, with clinical trials currently in progress for low-risk patients. However, adoption rates vary from country to country as a result of heterogeneous reimbursement programmes and high device costs. The clinical relevance of current risk scores is under question, and the recent ESC/EACTS guidelines on valvular heart disease1 have adopted a simpler classification into low risk and increased surgical risk – with ultimate procedural choice between TAVI and surgical aortic valve replacement (SAVR) being directed by a combination of age, anatomical and clinical factors (including comorbidity and frailty), and overall life expectancy. Despite extensive laboratory testing, the durability of current TAVI devices in vivo remains unknown, since the majority of early TAVI procedures were carried out in elderly patients at high and extreme risk. Meaningful long-term outcome data will not be available for some years to come, and routine use of TAVI in younger patients is not yet appropriate beyond the remit of randomised controlled trials.
Although TAVI is now a standardised and predictable procedure when performed by experienced teams, allowing the safe and effective treatment of many patients with symptomatic severe aortic stenosis (AS), there are still some important technical deficiencies. Although >90% of procedures can currently be performed via transfemoral approach, there will remain a need for alternative access routes until lower-profile devices make the transfemoral approach feasible in all patients. Permanent pacemaker (PPM) implantation rates remain high for some devices, an issue which will need to be addressed if TAVI is to replace SAVR in younger patients. For example, novel device designs with less radial force or limited extension into the left ventricular outflow tract have been associated with very low PPM rates. Bicuspid aortic valve (BAV) anatomy is more common in younger cohorts with AS and is associated with specific features which make TAVI more challenging, including increased (and often asymmetric) valve calcification and non-circular annular geometry (both of which increase the likelihood of paravalvular leak), and associated aortic root dilatation (which may make combined surgical valve and root replacement a more appropriate treatment option).
Specific devices which have high radial strength and are tolerant of ovoid inflow may be required for patients with BAV though these design features may have benefits for other patients. Alternative adjunctive technologies, such as cutting valvuloplasty balloons or lithotripsy devices, and modified delivery systems to improve alignment and minimise instrumentation of the ascending aorta, may prove beneficial, though maintained durability will be a key consideration in this younger patient population.
Concomitant aortic regurgitation (AR) is frequently encountered in the AS population and readily addressed using current TAVI devices providing there is sufficient annular calcium to ensure stable anchoring. However, patients with pure native AR and minimal calcification (particularly those with a large valve annulus) pose a significant challenge. The design features of some existing valves (such as stabilisation arches on the ACURATE neo valve and the ability to carry out a “tug test” to confirm stability with the LOTUS valve – both Boston Scientific, Marlborough, MA, USA) show promise in this patient group, while future devices could accommodate larger annular diameters (with improved sizing algorithms) and employ a high friction outer coating to improve stability and reduce the risk of embolisation.
The workshop participants concluded that: A) future TAVI devices will be cheaper, durable, and simple to use in most catheterisation laboratories by most interventional cardiologists, B) current geographical disparities in patient access to TAVI will reduce as commercial competition increases and more affordable valves come to market, and C) TAVI will soon become the standard of care for most patients with aortic valve disease, except in certain situations where open surgery retains a role, such as coexistent severe multivessel coronary artery disease, aortic root disease and infective endocarditis.
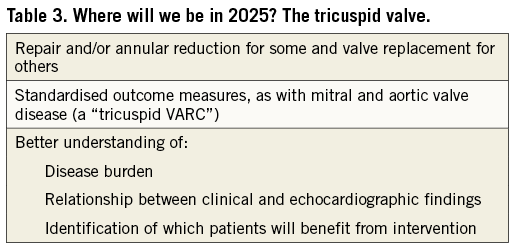
The mitral and tricuspid valves (Table 2, Table 3)

Percutaneous mitral valve intervention is a more complex area: treatment goals are yet to be defined, depending on the underlying disease mechanism(s). While percutaneous treatment of mitral stenosis has been established for decades (and is now the first-line treatment in most cases), less invasive techniques for the treatment of mitral regurgitation (MR) have only been developed in recent years (with mixed results). Is the overall aim to replace surgery, treating the same patients (mostly symptomatic severe primary MR) in the same way – implying that repair is preferable to replacement? Or could there be a role for percutaneous treatment earlier in the natural history of the disease? And how do we judge success? Is modest reduction in the severity of MR sufficient in the setting of a “safer” procedure? Or should we be aiming for abolition of MR in all patients – at the risk of inducing afterload mismatch?
Participants concluded that mitral repair will remain the preferred treatment, with a “toolbox” of different devices for different anatomical patterns. Transcatheter mitral valve replacement will be required in some patients (perhaps those with Barlow’s or rheumatic heart disease) but it seems unlikely that a “one size fits all” approach will be appropriate, given the heterogeneity of mitral valve disease.
Surgery is seldom undertaken for isolated secondary MR (unless coronary artery surgery is otherwise indicated) as a consequence of inconsistent clinical outcomes and a low-quality evidence base. These factors point to a potential future niche for transcatheter approaches. The eagerly anticipated results of upcoming randomised trials comparing percutaneous edge-to-edge repair with medical therapy will be important in determining the future of transcatheter intervention in such patients and procedural safety will be an essential focus. However, neutral or negative trial outcomes need not necessarily signal the end of the story. Our current approach to secondary MR – reserving intervention until late in the natural history of the disease (“like giving someone a parachute just before they hit the ground”) – may need to change. As transcatheter devices with a smaller anatomical footprint become easier to use, there may be a role for much earlier treatment if it can be demonstrated that this approach halts or delays progression of MR with associated improvement in clinical outcomes.
Tricuspid valve disease is another challenging field for percutaneous intervention and procedural imaging guidance poses particular difficulties. Unlike aortic and mitral valve intervention, previous experiences with surgery do not offer clear direction in the prediction of which patients may benefit from intervention; there is considerable scope for development in this area. Patient selection is difficult since quantification of tricuspid regurgitation (TR) and right ventricular function is difficult and predictors of clinical success are uncertain. Development of safe and effective percutaneous options for treatment of tricuspid valve disease may lead to lower thresholds for intervention while common definitions for the quantification of TR and standardised clinical endpoints will facilitate trial design. Accumulating experience over the coming years will enrich the evidence base and enable more robust guideline recommendations – although this may be a prolonged process.
Engineering and imaging (Table 4)
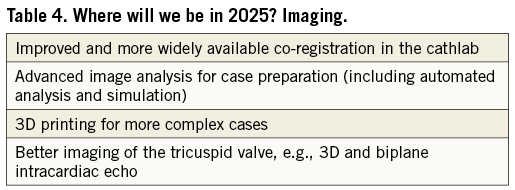
Engineering developments will ensure that percutaneous valve devices become smaller, safer and more effective, while concomitant improvements in imaging will be vital to ensure that mitral and tricuspid valve interventions become more predictable and simple. Increased integration of imaging specialists (“the eyes of the Heart Valve team”) and dedicated training for the next generation of interventionists in the principles of three-dimensional imaging will be key requirements2,3. The workshop participants concluded that co-registration of CT, echocardiographic and fluoroscopic data will allow mitral and tricuspid valve intervention to become mainstream therapies within the capabilities of most operators in most hospitals, rather than being limited to more specialist centres. Advances in CT analysis, simulated implantation and 3D printing will improve preprocedural planning, particularly in more challenging anatomy and unusual cases. Devices with fixed elements (such as the delivery system) and personalised elements (such as the valve leaflets or anchoring system) may soon become a reality.
While we are accustomed to learning about novel devices and emerging research from clinicians, PCR London Valves Innovators Day 2017 provided a refreshing and thought-provoking opportunity to hear the perspectives of less frequently encountered individuals who drive product development – such as the engineers and animal laboratory specialists – and brainstorm ideas for future development with industry leaders (selected presentations from PCR London Valves Innovators Day 2017 are available at PCR Online [www.pcronline.com]). 2017 marks the fortieth and fifteenth anniversaries of angioplasty and TAVI, respectively. These are exciting times for valve intervention, and the PCR London Valves Innovators Day programme offered a fascinating insight into developments we can expect in the coming years.
Acknowledgements
The authors wish to thank all those who contributed to the success of PCR London Valves Innovators Day 2017, in particular those who participated in the round table discussions facilitated by the following individuals: Andreas Baumbach, James Eadie, Farrel Hellig, Pieter Kappetein, Azeem Latib, Ian Meredith, Martin Rothman, Lars Sondergaard, Martyn Thomas, and William Wijns.
Conflict of interest statement
The authors have no conflicts of interest to declare.

