Abstract
Aims: Intimal hyperplasia, characterised by smooth muscle cell migration and proliferation, requires extracellular matrix degradation which is mediated by matrix metalloproteinases (MMPs). In this study, the effect of tetracycline derived doxycycline, a specific MMP inhibitor of both activity and synthesis, on intimal hyperplasia in vitro and in vivo was assessed.
Methods and results: Segments of human saphenous veins were cultured for 4 weeks in absence or presence of doxycycline (10µg/ml) (n=6). A 81% inhibition in intimal hyperplasia was observed in the doxycycline treated segments compared to controls. To assess the effect of doxycycline on intimal hyperplasia in vivo, perivascular cuffs were placed around femoral arteries in mice with or without doxycycline in the drinking water (3 mg/ml). In this in vivo model for intimal hyperplasia doxycycline significantly reduced (68%, n=6) intimal hyperplasia when compared to controls.
In addition the effect of doxycycline on vein graft thickening was assessed in a murine venous interposition model. In this in vivo model vein graft thickening was reduced by 35 % in the doxycycline treated mice (3 mg/ml in drinking water). Furthermore, a reduction in vascular MMP expression was observed in these mice.
Conclusion: Treatment with tetracycline derived doxycycline results in significant inhibition of intimal hyperplasia in vitro and in vivo and may be an effective strategy to prevent post interventional restenosis and vein graft disease.
Introduction
Neointima formation plays an important role in the pathogenesis of atherosclerosis, restenosis after angioplasty, and late vein graft failure1. In the process of neointima formation, smooth muscle cells (SMCs) migrate from the media across the inner elastic lamina to the intima2,3. This migration of SMC requires degradation of basement membrane and extracellular matrix surrounding the cell by matrix metalloproteinases (MMPs)4-7. The role of MMPs in SMC migration is underlined by the elevated MMP levels, observed after arterial injury4,5,7-9, after PTCA in patients10 and in pig vein bypass graft models11,12. Also, inhibition of MMPs by synthetic MMP inhibitors impaired SMC migration in various in vitro and in vivo models13-17. Therefore, MMP inhibition is a promising approach in the prevention of neointima formation and vein graft disease.
Tetracyclines and tetracycline derivatives such as doxycycline are able to inhibit MMP activity, independent of their anti-microbial action18. The inhibitory capacity of tetracyclines differs for various MMPs: it is the strongest for MMP-8 and MMP-13, and only weak or absent for MMP-1 and MMP-3. Tetracyclines can not only inhibit the enzyme activity of MMPs, but also affect the activation of MMPs and in particular MMP synthesis at the mRNA level. An additional advantage of doxycycline is the specificity of this effect for a limited number of MMPs, in contrast with broad range MMP inhibitors. No effect of tetracyclines on the synthesis and action of TIMPs, the natural inhibitors of MMPs, was observed. Tetracyclines may therefore be used as inhibitors of MMP synthesis and activity in vivo, without the side effects such as tendinitis as observed in clinical trials using synthetic MMP inhibitors.
The sensitivity of various MMPs to these additional effects of tetracyclines also differs per MMP. For a number of MMPs (MMP-8, MMP-9 and MMP-13) inhibition occurs at levels which are physiologically obtainable upon therapy19.
In the present study, as a step towards application of tetracycline based MMP inhibition in the prevention of intimal hyperplasia and vein graft disease in patients, we evaluated the effect of doxycycline, as a specific tetracycline based MMP inhibitor, on neointima formation in a saphenous vein organ culture model in vitro. Furthermore, we analysed in vivo the effects on cuff-induced neointima formation, and on vein graft thickening in mice.
Materials and methods
In vitro human saphenous vein organ cultures and analysis of neointima formation
Segments of human saphenous vein were obtained from patients undergoing coronary bypass graft operation, according to the guidelines of the local ethical committee. The veins were cultured for 4 weeks using a modified method as described previously20,21. From each patient, one segment served as control, one segment was incubated with doxycycline (10 µg/ml) and one segment incubated with Marimastat (10 µg/ml). Vein segments were cultured for 4 weeks and analysed histochemically. Neointima formation in the treated segments was always compared with their untreated counterparts and quantified on multiple segments (n=6 patients) at standard magnification, at least 6 sections per segment, using Qwin image analysis system (Leica).
In vivo femoral artery cuff placement
All animal experiments were approved by the Animal Welfare Committee of TNO. Male C57BL/6 mice (20-25 gr) were anesthetised with Hypnorm (Bayer) and Dormicum (Roche) (25 mg/kg i.p. each). The left femoral artery was isolated from surrounding tissues, loosely sheated with a 2.0-mm polyethylene cuff (PE-50 tubing, inner diameter 0.4 mm, Becton Dickinson) as described previously21,23. One group of mice (n=6) received doxycycline in their drinking water (3 mg/ml) after cuff placement while the other group (n=6) served as controls. Animals were sacrificed after 21 days. After perfusion fixation tissue segments were paraffin-embedded. Weigert’s elastin staining was used to visualize elastic laminae. Neointima formation was quantified using image analysis in 6 representative serial sections per vessel segment.
In vivo vein graft procedure
To assess the effect of MMP inhibition by doxycycline in vein graft thickening, FVB mice were anesthetised with Hypnorm (Bayer, 25 mg/kg) and Dormicum (Roche, 25 mg/kg) i.p. Atropine sulfate (1.7 mg/kg body weight) was administered to maintain the respiratory tract in good condition. The procedure used for vein grafts was similar to that described by Zou et al.22. In brief, the right common carotid artery was dissected free from its surrounding from the bifurcation at the distal end towards the proximal end. The artery was cut in the middle and a cuff placed at the end on both sides. Next, the artery was everted over the cuff and ligated with an 8.0 silk ligature. The caval vein was harvested and grafted between the 2 ends of the carotid artery by sleeving the ends of the vein over the artery cuff and ligating them together with an 8.0 silk suture. Mice were randomised in two groups. One group (n=6) received doxycycline (3 mg/ml) in their drinking water directly after surgery, while the other group served as controls receiving normal drinking water.
Mice were sacrificed 28 days after surgery for histological analysis. After perfusion fixation, the vein graft was harvested; fixated overnight in 3.7% formaldehyde in phosphate buffered saline and paraffin-embedded. Six equally spaced cross-sections throughout the center of the graft were used in all mice to quantify intimal lesions. Using image analysis software (Qwin, Leica), total cross sectional area was measured between the lumen and the adventitia. All samples were routinely stained with hematoxylin-phloxine-saffron (HPS).
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded sections. Polyclonal antibodies against human MMP-2 and MMP-9 (raised in our laboratory) were used to detect MMP-2 and MMP-9. After incubation with an Donkey anti Rabbit biotin (Vector) and avidin-biotin complex (ABC,Dako) antibodies were visualised with Novared (Vector).
Gelatin zymography
Gelatinolytic activities of secreted MMPs were analysed by zymography on gelatin-containing polyacrylamide gels. Using this technique both active and latent gelatinases can be visualized. Arterial samples from three aortas from mice in each treatment group (normal drinkwater vs doxycycline) were freeze-dried, pulverized, and resuspended in MMP assay buffer (50 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 5 mmol/L CaCl2, 1 µmol/L ZnCl2, and 0.01% [vol/vol] Brij-35) at 4°C. After centrifugation at 13 000 rpm samples were resuspended in 2% (w/v) SDS and 10% (v/v) glycerol and applied to 10 % (w/v) polyacrylamide gels co-polymerized with 0.2% (w/v) gelatin. Conditioned medium of HUVEC cultured in M199 supplemented with 0.03% human serum albumin and with or without 10 nmol/L PMA was used as a control. After electrophoresis the gels were washed twice for 15 min in 50 mmol/L Tris/HCl, pH 8.0, containing 5 mmol/L CaCl2, 1 (mol/L ZnCl2 and 2.5% (w/v) Triton X-100 to remove the SDS, followed by two washes of 5 min in 50 mmol/L Tris/HCl, pH 8.0, containing 5 mmol/L CaCl2, and incubated overnight at 37 °C. The gels were stained with Coomassie Brilliant Blue R-250.
Statistical analysis
All data are presented as mean±SD. Overall comparisons between groups were performed with the Kruskal-Wallis test. If a significant difference was found, groups were compared to their control using 2 tailed Mann-Whitney rank sum tests. P-values less than 0.05 were regarded significant.
Results
Effect of doxycycline on neointima formation in human saphenous vein organ cultures
In human saphenous vein organ cultures, a multilayer neointima is formed in 4 weeks that consists mainly of α-SMC actin positive cells. These cells originate from the media and the adventitia and migrate towards the luminal side of the vein to form the neointima. Neointima formation was studied in the absence or presence of doxycycline in the culture medium. A significant reduction in neointima size was observed in all the studied sections (Figure 1A). In the control sections, after 4 weeks a multilayer neointima was observed whereas in the sections of doxycycline-treated organ cultures a strong inhibition of neointima formation was observed, the neointima was comparable to segments harvested at the start of the culture. Neointima formation was quantified by image analysis in multiple sections (n=6) of the segments, pair-wise in treated (doxycycline) and untreated counterparts (Control) (Figure 1B). The mean neointimal area was 0.187±0.07 mm2 in the control segments and 0.035(0.05 mm2 in the doxycycline treated segments. (Mean±SD, p<0.05). The mean inhibition of neointima formation was 81%. As a control a synthetic MMP inhibitor was used (marimastat) and resulted in a mean intima area of 0.0494±0.04 mm2 (74% inhibition).
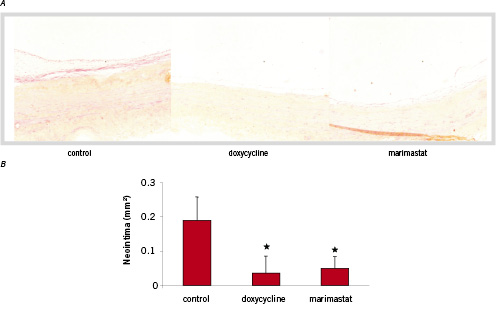
Figure 1. HPS staining on representative cross sections of saphenous vein organ cultures. In the control section smooth muscle cells have migrated from the media and the adventitia to form a neointima (magnification 100x). Doxycycline and Marimastat treated segments show a reduction in neointima formation. B. Quantification of saphenous vein organ cultures (n=6) by image analysis. Area is expressed in mm2 (mean ± SD) *p< 0.05.
Effect of doxycycline on cuff-induced neointima formation in mice
In order to analyse the effect of doxycycline-mediated MMP inhibition on neointima formation in vivo, we have used a model for cuff-placement induced neointima formation in C57Bl6 mice. Cuff placement resulted in the formation of a neointima within 3 weeks predominantly consisting of αSM-actin positive SMCs underneath a layer of von Willebrand Factor-positive endothelial cells as described previously18 in mice on normal drinking water (figure 2A). Quantification of the total neointima area revealed a significant inhibition of neointima formation of 68% in doxycycline treated mice compared to control mice (8746±3891 vs 2753±2642 µm2, p<0.05) (figure 2B). No significant change in medial area was observed between groups. This resulted in a reduction of neointima/media ratio of 68% in mice on doxycycline compared to control mice.
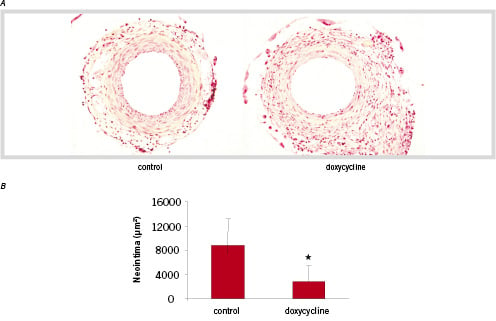
Figure 2. Crossection of cuff induced neointima inC57Bl6 mice 21 days after surgery. A significant decrease in neointima formation is seen in the doxycycline treated mice. (HPS staining, magnification 100x) B. Quantification of the cuff induced neointima. Areas were quantified by image analysis using six serial sections per mice (n=6) and are expressed in µm2 (mean ± SD) *p< 0.05.
Effect of doxycycline on vein graft thickening in mice
To characterize the effect of doxycycline on intimal hyperplasia in vein graft disease, FVB mice were randomised in 2 groups upon vein graft surgery. One group (n=6) received doxycycline in their drinking water directly after bypass surgery, and one group (n=6) was kept on normal drinking water after placement of the graft. Twenty-eight days after surgery, light microscopy of transverse sections through the vein grafts revealed that in the mice on normal drinking water a thickening of the vessel wall to approximately 10 cell-layers thick occurred, while proximal and distal segments of carotid artery possessed normal histology (figure 3A). This intimal thickening consisted predominantly of αSM-actin positive cells, although monocytes/macrophages were also detected (Data not shown). An intact endothelial layer was observed in the grafts 28 days after surgery (Data not shown). In the group of animals on doxycycline, a profound decrease in vessel wall thickening compared to control mice was observed (figure 3A). Quantification of total intimal areas revealed a significant inhibition of vessel wall thickening of 35% in mice on doxycycline compared to control mice (0.3076±0.04 vs 0.2007±0.04, p<0.01) (figure 3B). No significant change in mean vein graft circumference was observed between groups. In doxycycline-treated mice an increase in total luminal area was observed compared to control mice, although significance was not reached.
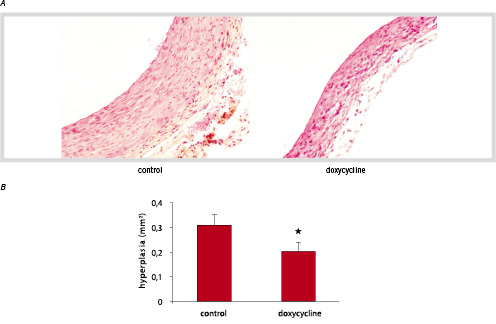
Figure 3. Crossections of murine vein grafts 28 days after surgery. B. Quantification of vein graft hyperplasia in control mice (n=6) and doxycycline treated mice (n=6) (HPS staining, magnification 150x). Hyperplasia was measured between the lumen and adventitia (mean ± SD) *p< 0.05.
MMP inhibition in the vessel wall in vivo
In order to demonstrate the effects of doxycycline on MMPs in vivo, vessel wall segments of doxycycline treated mice and their untreated counterparts were analysed for MMP-2 and MMP-9 by immunohistochemistry and zymography on tissue extracts. In both the control and doxycycline treated cuffed femoral arteries MMP-2 expression is visible in the neointima. In the media, MMP-2 expression can only be observed in the control group. In the cross-sections of the cuffed femoral arteries a clear reduction of MMP-9 can be observed in the intima and media of the doxycycline treated vessel wall (Figure 4A). In cross-sections of the murine vein grafts MMP-2 expression is abundant in the hyperplasia. In both groups MMP-9 expression can be observed in activated endothelial cells and in macrophages or leucocytes in the surrounding tissue. Almost no MMP9 staining is visible in the intimal hyperplasia of the doxycycline treated group (Figure 4B). The MMP inhibitory activity of doxycycline in vivo was assessed in tissue extract of the aorta of mice (n=3 per group) either or not treated with doxycycline by zymography. MMP-2 inhibition by doxycycline can be observed. In both groups no MMP-9 expression can be observed, this could be explained by the finding that MMP-9 is only expressed in activated cells. The observed reduction in MMP-2 in the doxycycline-treated aortas demonstrated that doxycycline treatment in vivo functionally affects MMPs (Figure 4C).
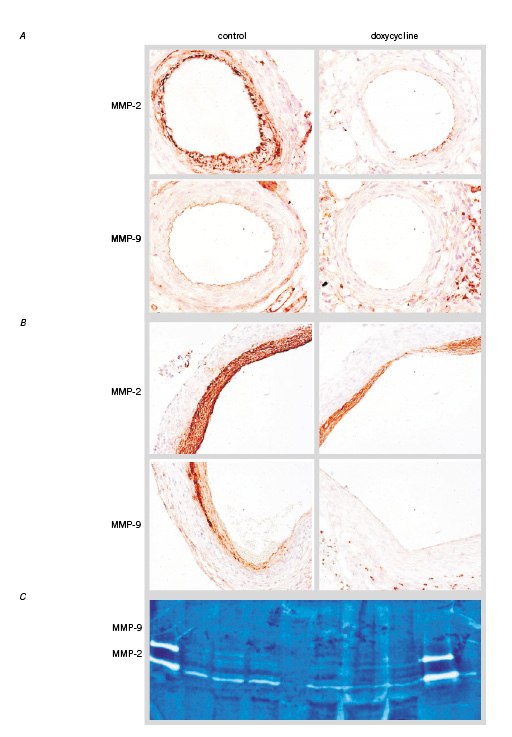
Figure 4. A. MMP staining on cuff induced neointima formation. In the control section is an abundant MMP-2 expression in the smooth muscle cell rich neointima and in the media visible. In the doxycycline treated mice there is MMP-2 staining in the neointima but no staining in the media. MMP-9 is expressed in the endothelial cell lining in the control section. There is almost no staining visible in the doxycycline treated section (magnification 100x). B. Vein graft thickening. MMP-2 expression in both the control and doxycycline treated sections whereas the staining in the doxycycline treated mice is less abundant. MMP-9 staining in macrophage or PMN’s and endothelial cells in both sections. Almost no MMP-9 staining in the SMC layer (magnification 100x). C. Zymogram of control (left lanes) and doxy treated (right lanes) (n =3) aortas. A slight reduction of MMP-2 is visible. In both groups no MMP-9 is detectable.
Discussion
The principal finding of the present study was that doxycycline significantly reduced neointima formation in vein graft disease in vitro and in vivo, and that this effect is most likely mediated via inhibition of MMPs.
That increased metalloproteinase activity may contribute to arterial pathology is seen in aneurysmal degeneration24, atherosclerotic plaque formation and disruption25,26 and neointima formation after angioplasty and surgical reconstruction27,28. The increased MMP activity after vascular injury alters the proteolytic balance, enhances SMC migration and matrix degradation and contributes to pathological changes in diseased vessel segments29. Therefore, modulation of MMP activity holds many promises in preventing neointima formation and thereby controlling cardiovascular diseases. A number of specific synthetic MMP inhibitors have recently been developed and although pre-clinical studies showed promising results, clinical application is hampered by unacceptable side-effects33.
Doxycycline belongs to the family of tetracyclines. These groups of antibiotics have proven long term safety, are used for many months to years in low doses and have good side-effect profiles30,31. Beside its anti-microbial activity, doxycycline is able to inhibit both MMP activity and MMP expression of several MMPs such as MMP8, MMP9 and MMP13 in vitro and in vivo. To date, doxycycline with its favourable pharmacokinetics and excellent tolerability appears one of the most promising, registered MMP inhibiting drugs.
Here, we evaluated the effect of doxycycline on neointima formation in vitro in a human saphenous vein organ culture model and in vivo in two murine models.
In this study, treatment with doxycycline significantly reduced neointimal thickness in a human tissue culture model of vein graft stenosis similar as has been reported by others34 suggesting a direct role for MMPs in vein graft stenosis. This finding is in accordance with recent data showing elevated levels of MMPs localised to the internal elastic lamina of cultured saphenous vein segments and in vein grafts in vivo suggesting a role of MMPs in facilitating SMC migration to the intima35. Also, the reduction of MMP activity by treatment with broad-spectrum MMP-inhibitor marimastat resulted in significantly reduced neointimal thickness in an in vitro model for arterial intimal hyperplasia36,37. This underlines the role of MMPs not only in venous intimal hyperplasia but also in human arterial neointima formation. Furthermore, MMP inhibition suppressed SMC migration in human in vitro experimental models38-41. Also, overexpression of inhibitors of metalloproteinases resulted in inhibition of SMC migration and neointimal formation in in vitro human saphenous vein culture model42 and inhibition of intimal hyperplasia in a rat carotid injury model and rabbit carotid injury model43-46. On the other hand, overexpression of MMP enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery47.
To study the effects of doxycyline in vivo, we induced intimal hyperplasia by placement of a non-constrictive cuff in C57bl/6 mice. Immunohistochemical staining of MMPs in these cuffed femoral arteries shows an upregulation of MMP-2 in the neointimal area after cuff placement. A similar increase of MMP has been described for neointima formation induced by balloon denudation in rat carotid arteries 4 suggesting a correlation between overexpression of MMPs and increased vascular smooth muscle cell migration in vivo12. In our study, treatment with doxycycline resulted in significant inhibition of intimal thickening and down-regulation of MMP expression in this experimental model. These in vivo data suggests that inhibition of MMPs by doxycycline reduces migration of SMCs from the media to the neointima.
To assess the effect of doxycycline in an animal model mimicking more the clinical situation of vein graft failure, the murine vein graft model previously described by Zou et al. was used48. Previous studies describe that MMP-9 is undetectable in veins but significantly upregulated in early vein grafts8. This upregulation may be explained by altered mechanical circumpherential and shear stresses within the graft49,50. We observed that in vivo inhibition of both MMP-9 activity and expression by doxycycline reduced vein graft thickening significantly. This finding suggest a central role of MMPs in neointima formation in vein graft disease, which is consistent with the observed inhibition of neointima formation by overexpression of TIMP-3 in late porcine vein grafts51.
Recently, the effect of doxycycline on cardiac events was assessed in patients after percutaneous coronary intervention52. In this non-randomised study with relatively low dose of doxycycline and relatively short follow-up of 6 months, subgroup analysis revealed a beneficial effect of doxycycline in non diabetic male smokers. The low clinical event rates founding on relatively low follow-up rates in turn bring about a low test power resulting in uncertainty about the beneficial effects of doxycycline in patients.
The results of the present study, in which we studied three different models of neointima formation, indicated that 1) MMP expression is closely related to induction of intimal hyperplasia in vitro and in vivo and that 2) inhibition of upregulated MMP levels after vascular injury may be an effective strategy to prevent post interventional neointima formation and vein graft disease. 3) doxycycline may be applied as a selective MMP effecting drug without the, for the synthetic MMP inhibitors such as Marimastat, described side-effects.
Acknowledgement
This study was performed with financial support of the Molecular Cardiology Program of the Netherlands Heart Foundation, grant no. M 93.001.

