Abstract
Aims: Iatrogenic ascending aorta dissection (AAD) is an uncommon complication following heart catheterisation. Aims of this study were to determine the incidence, to identify the predisposing procedural factors and to define the management and the outcomes of patients suffering from iatrogenic AD.
Methods and results: Between January 1996 and May 2005, iatrogenic AAD that occurred during cardiac catheterisations, were retrospectively identified from clinical and peri-procedural data prospectively collected in a dedicated database. At least 1-month clinical follow-up (median 25 months; range 5-77) was obtained in all patients complicated with iatrogenic AD. The overall incidence of iatrogenic AAD was 0.04%; this incidence was significantly higher after interventional procedures (0.12%) than after diagnostic procedures (0.01%; p=0.0001). Most of dissections were located in the right coronary sinus (12 patients; 67%) and limited to the corresponding coronary sinus (Dunning class I: 11 patients; 61%). Manoeuvres most often involved were coronary engagement with the use of non-conventional catheters. Conservative treatment with sealing the entry door by a stent 10 patients (56%) or expectant management 7 patients (39%) resulted in favourable outcomes as none of patients died during hospitalisation and follow-up.
Conclusions: Iatrogenic AAD is a rare complication following cardiac catheterisation that in the vast majority of patients may benefit from conservative treatment with good long-term outcomes.
Introduction
Iatrogenic dissection of the ascending aorta (AAD) is an uncommon and severe complication during heart catheterisation. The incidence ranges between 0.02% and 0.08% in diagnostic procedures and between 0.07% and 0.6% during percutaneous coronary interventions (PCI)1-11. The main mechanism involved is retrograde extension of a coronary artery dissection into the aortic root9. Clinical presentation and outcome of iatrogenic AD seem to differ from those of spontaneous AD8. There is little information about iatrogenic AD and it is also controversial what the best approach to manage it is. The aim of this study was to determine the incidence and to identify predisposing procedural factors, management, and outcome of AD complicating heart catheterisation.
Methods
Patient selection
From January 1996 to May 2005, 28,869 diagnostic procedures and 12,031 PCI were performed in our institution. Since 1996 data from every procedure are prospectively collected in a dedicated database (Apolo, Palex Inc), in which any complication during or after the procedure is specifically entered.
Definitions
Iatrogenic AAD was defined as a dense staining of the aortic wall with incomplete dye clearance after the injection of contrast media that occurred during coronary catheterisation. To define the extension of the dissection, we used the classification proposed by Dunning et al9 that categorised iatrogenic dissections into 3 classes (Table 1).

For the purpose of the study, patients with other causes of AAD, such as spontaneous or traumatic AAD and those dissections originating from femoral or iliac vessels were not considered eligible.
Study design
This is a retrospective study from prospectively collected data. Procedural details reported in database and cinefilm/CD recordings from every procedure complicated with iatrogenic AAD were reviewed by 2 experienced interventional cardiologists. Demographic, angiographic and procedural variables from the database were retrospectively analysed. Besides, image techniques used and therapeutic interventions performed following this complication were also evaluated. The additional image techniques in order to evaluate the extension of the iatrogenic AAD were selected at the discretion of the operator and included transthoracic echocardiography, transesophageal echocardiography, magnetic resonance imaging, thoracic computed tomography and intravascular ultrasound. A clinical follow up was carried out to assess short (1 month) and long-term outcome (>1 year).
Statistical methods
Data is presented as percentages or mean±standard deviation. To compare the incidence of iatrogenic AAD between diagnostic and therapeutic procedures the Fisher’s exact test was used. A two-tailed value of p < 0.05 was considered statistically significant.
Results
Incidence and baseline characteristics
From January 1996 and May 2005, 18 cases of iatrogenic AAD were identified; 15 during PCI procedures and 3 during coronary diagnostic catheterisation. The incidence was significantly higher after PCI than in diagnostic procedures (0.12% vs. 0.01%, p=0.0001). All procedures were performed by a femoral approach. Eleven patients were male (61%) and mean age was 63±8 years. Nine patients (56%) had undergone a previous uncomplicated coronary angiography procedure and 5 of them (28%) a previous PCI procedure. Prior history of myocardial infarction was present in 50% and prior history of cardiac surgery in 3 patients (17%). Catheterisation was indicated due to unstable angina pectoris in 8 patients (44%), acute myocardial infarction in 7 (39%) and stable angina pectoris in 3 (17%). Multivessel disease was identified in 5 patients (28%) whereas chronic total occlusions were identified in 7 patients (39%), 5 of them (28%) in the right coronary artery (RCA).
Iatrogenic AAD location and extension
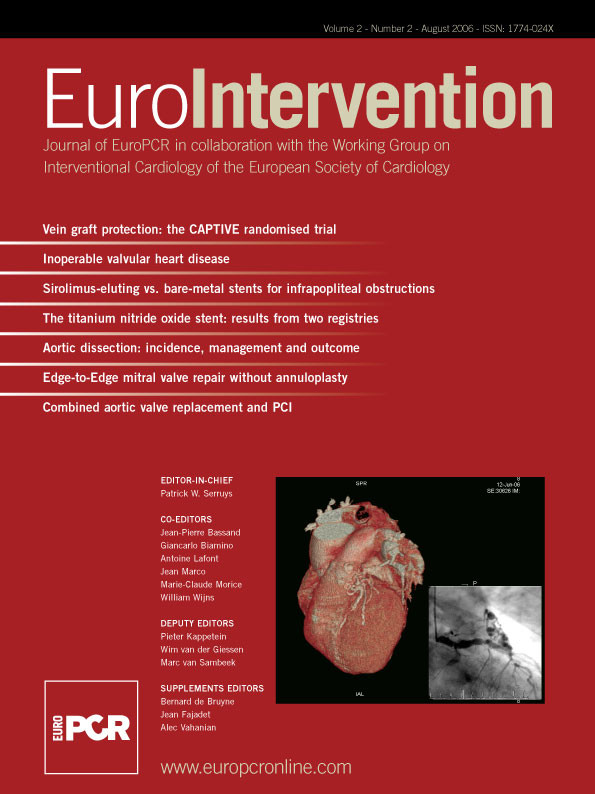
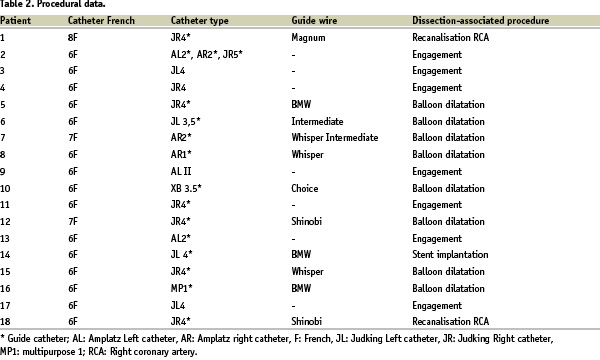
Right coronary sinus was most often involved (12 patients; 67%). According to the classification proposed by Dunning et al, there were 11 patients with class I dissections (limited to the Valsalva sinus), 4 patients with class II (<40 mm in the ascending aorta) and 3 patients with class III (extending >40mm in the ascending aorta). Examples of each type are depicted in figures 1 to 3.
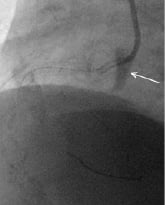
Figure 1. Dunning type I iatrogenic aortic dissection localised into the right aortic coronary sinus.
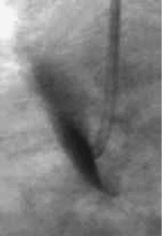
Figure 2. Dunning type II iatrogenic aortic dissection.
Figure 3. Dunning type III iatrogenic aortic dissection.
Additionally, in 9 cases (50%) the dissection also progressed in anterograde direction distal into the coronary artery. Dunning class III dissections were more often associated with exclusive retrograde extension of the AD as compared with those classes I and II AD (61% vs. 27%; p=0.2).
Procedural data (table 2)
After careful review of the angiogram report, the technician’s worksheet and the cinefilms/CD, the manoeuvre involved in the occurrence of the AD could be identified in all patients. Therefore, in 7 patients AD occurred during coronary engagement; balloon dilatation was causing the complication in 8 patients, during an attempt to cross a total chronic occlusion of the right coronary artery in 2 patients, and stent implantation in 1 patient. Neither rotational atherectomy nor a cutting balloon was used in any patient. Non-conventional catheters (Amplatz, XB, MP 1) were used in 7 patients (39%). The instrumentation used in each patient is described in table 2.
Management and in-hospital outcomes
A coronary entry of the dissection could be identified in 10 patients (56%) on angiography. Usually, the entry was located at the tip of the catheter for those dissections that occurred during engagement or at the place of the balloon dilatation or stent implantation (table 2). This entry could be sealed with coronary stent implantation in all of them. Nine of them did not require further interventions but 1 patient required emergency surgical intervention due to acute partial occlusion of the stent implanted in left main artery. In this patient, a double saphenous vein graft was performed to the left anterior descending and left circumflex coronary arteries but no specific therapy was selected to tackle the aortic dissection. A postoperative transthoracic echocardiography showed intramural haematoma (IMH) extending from the Valsalva sinus to the beginning of the aortic arch. Before discharge, nearly complete resolution of aortic haematoma was evidenced on transthoracic echocardiography. Another patient, in whom the entry site was sealed with a stent, suffered from convulsive crisis due to the extension of the dissection up to the level of the left subclavian and left vertebral arteries, right brachyocephalic trunk, the carotid bulb, and the right common carotid artery. After this acute event the patient recovered ad integrum. Following a careful evaluation for both vascular and cardiac surgeons, this patient was initially managed in a conservative way, did not experienced further complications and was discharged 32 days after the event. Aortic dissection was resolved before discharge in serial magnetic resonance imaging.
Seven patients were initially managed without any intervention. All of them had type I aortic dissection and presented a favourable outcome being discharged from the hospital without complications. Of these, 2 patients underwent further elective cardiac surgery intervention to treat their severe coronary lesions 25 and 180 days after occurrence of iatrogenic AAD, respectively.
Finally, 1 patient in cardiogenic shock secondary to left main coronary dissection extended into the aortic root underwent an urgent surgery and had also favourable in-hospital outcome.
Additional diagnostic techniques
Additional diagnostic techniques were used at the discretion of the operator. At least 1 technique was additionally performed in all patients. Transthoracic echocardiography in 10 patients, magnetic resonance imaging in 5, transoesophageal echocardiography in 8, thoracic computed tomography in 1 and intravascular ultrasound in 1 patient. Supra-aortic angiography was performed in one patient with neurological deficit. In one patient, neither transoesophageal echocardiography nor magnetic resonance imaging detected any significant abnormalities in the aortic wall, and aortic dissection was only recognized with intravascular ultrasound. This technique also visualised the sealing of the entry site with a stent. Magnetic resonance imaging also failed to detect the dissection in another patient. In both patients, the AD was limited to the right sinus of Valsalva (Dunning type I). In 5 of the 18 patients (28%), an IMH was evidenced. This was finally resolved during serial imaging studies in all patients. An example of iatrogenic AAD visualized on transoesophageal echocardiography and magnetic resonance imaging are depicted on figures 4 and 5.
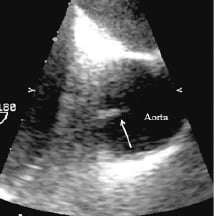
Figure 4. Transoesophageal echocardiography of an iatrogenic aortic dissection. The arrow indicates an intimal flap into the aorta lumen.
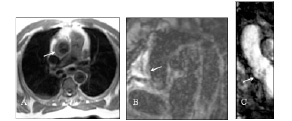
Figure 5. Magnetic resonance imaging of a Dunning type III iatrogenic aortic dissection.
Long term follow up
All patients that were discharged from the hospital reached at least 1 month follow-up (median 25 months; range 5-77 months). One patient died 18 months after the event due to a non-cardiac cause (end-stage cancer), and 2 patients needed a new target vessel revascularisation due to severe in-stent restenosis. To date, not any further event has been reported.
Discussion
Incidence and predisposing procedural factors
Iatrogenic AAD is a rare complication following cardiac catheterisation procedures as only occurred in 0.04% of overall procedures. The incidence was found to be higher after PCI than after diagnostic procedures and comparable to other reported series.
The manoeuvre directly related to this complication was more often a deep coronary engagement. Under such circumstance, especially whenever pressure damping is recognized, the injection of a large amount of contrast material should be avoided and the catheter should be quickly withdrawn12. Non-conventional catheters (Amplatz, XB, MP 1) were used in almost half of the complicated patients. Amplatz catheters are often selected when difficulties with catheter engagement with the use of standard preformed Judkins catheters are encountered. Cannulation of right coronary artery (67%) and treatment of chronic total occlusion (39%) were also related to this complication. In this regard, a tethering effect induced by an occlusive atherosclerotic lesion on the vessel layers could favour the development of the iatrogenic AD13.
The International Registry of Aortic Dissection reported 34 cases of iatrogenic AD, 19 of which were following cardiac surgery, 14 following coronary angiography or coronary intervention and, 1 following renal angioplasty8. In this registry, cardiac surgery was the most common cause of AD located in the ascending aorta, whereas, distal dissection was more likely to follow cardiac catheterisation. Compared to our findings, known atherosclerosis and prior history of cardiac surgery were predisposing factors often involved with this complication.
Management
Although this complication can be life-threatening, the decision for urgent surgical reparation was taken in relatively few cases. In our study, 2 patients (11%) required urgent surgery due to acute stent thrombotic occlusion and the rapid development of cardiogenic shock following complication but with favourable outcomes. In another 2 patients elective surgery was performed to treat the underlying coronary disease. In previous reported series, the need for urgent surgery ranged between 22% and 25%8,9.
The acute treatment of iatrogenic AD has not been clearly established. Sealing of the entry site by a stent appeared to be a very appealing way to solve the problem as it was technically feasible and fast14. In this regard, although the mechanism of iatrogenic AAD is still unclear, the entry site is usually a subintimal dissection in a coronary artery caused by the guiding catheter, the guide wire, the balloon or the stent. The injection of contrast into the subintimal space may induce a retrograde extension of the dissection into the aortic root if an anatomical resistance impedes the anterograde extension of the coronary dissection (i.e. calcium; stent strut). We used such an approach in 10 patients resulting in a complete resolution of the complication in 9 of them. As mentioned above, the remaining patient required urgent surgery due to stent thrombosis. Of interest is the fact that none of the class III iatrogenic AD required surgical repair and the outcomes were favourable.
For those cases with IMH we decided to keep the initial conservative approach with close image monitoring. No general consensus exists on the appropriate treatment of ascending aorta IMH. Some groups believe that the therapeutic strategy for this complication should be the same as that for aortic dissection. In a meta-analysis15, mortality of type A IMH treated medically was high, 56%. However, Asian series16-17 showed low death rates in proximal IMH treated medically (6% to 10%). Kaji et al16 showed that medical therapy with timed surgical repair in cases with progression can also be a rational therapeutic strategy. Song et al17 5 reported that 64% of medically treated type A IMH evolved without complications; most of them exhibited complete IMH resorption. The evolution of type A IMH appears to be more benign than that of classical dissection. Several factors such as maximum aortic diameter, IMH thickness, age, and pericardial or periaortic blood extravasation have been related to poor outcomes15-18. None of those factors were present in our patients.
Prognosis
At long-term follow-up, the overall mortality rate was 0%. The anatomical characteristic of the Valsalva sinus that tends to contain the dissection below the sinotubular junction may be related to this favourable outcome. It has been reported7,19 that aortic sinus dissections that remain localised during catheterisation tend to spontaneously resolve in the first month. In contrast to what happens in spontaneous AD, iatrogenic AD presents a retrograde direction12. In such a scenario, blood pressure is not pulsating favouring rapid resolution of such dissections.
Additional diagnostic procedures
Additional diagnostic imaging techniques may fail to detect iatrogenic AAD, especially in the event of class I dissections. This occurred in our series in 2 cases by the use of magnetic resonance imaging (in one of them transoesophageal echocardiography did not detect the tear that was only visible on intravascular ultrasound imaging). The diagnosis of iatrogenic AD was more difficult to establish than spontaneous AD in the International Registry of Aortic Dissection8 due to the atypical clinical presentation and a relative lack of the classical signs evidenced in imaging techniques. One of the hypotheses that have been considered is that iatrogenic aortic dissections occurs more commonly in the form of an intramural haematoma than do spontaneous AAD. Furthermore, iatrogenic AD tends to be more localised such that flow is not evident in the small false lumen. In this regard, 5 of our patients (28%) were diagnosed of intramural haematoma.
Limitations
This is a retrospective study made with data collected prospectively. Small class I iatrogenic AAD may be overlooked by the use of angiography and only be seen on intravascular ultrasound10. Thus, their incidence may be eventually underestimated. However, the use of intravascular ultrasound is not a routine technique used during heart catheterisation. We have not included those iatrogenic dissections originating from the femoral or iliac arteries that have different clinical implications and dissections related to cardiac surgical procedures. Finally, due to the retrospective nature of the study we cannot draw the conclusion that surgical repair does not play a role in the management of this complication. This should be explored in randomised trials, although the low incidence of this complication makes this scenario to difficult to be realistic.