Introduction
The use of a non-permanent versus temporary/degradable implant to treat coronary vascular disease has been an attractive concept since the advent of percutaneous coronary revascularisation with balloon angioplasty and stenting. An ideal bioresorbable stent would provide mechanical support to the blood vessel wall for a sufficient period of time and bioerode into components which are eliminated from the human body leaving behind an open and functional blood vessel. Bioresorbable polymeric stents can release anti-proliferative or anti-inflammatory drugs to control intimal hyperplasia caused by the interventional procedure. These stents would be MRI compatible, could facilitate re-intervention by percutaneous or surgical means and may also have the potential to reduce late stent thrombosis due to the absence of a permanent implant.
One of the challenges in developing a polymeric resorbable stent is its ability to provide sufficient strength to the vessel over a sufficient period of time. In addition, ease of use of the bioresorbable stents should be similar to conventional metallic stents as well.
The Igaki-Tamai® stent1 was the first fully bioresorbable stent to be used clinically, and was made from poly(L-lactide) or PLLA. The PLLA stent required heating to 50°C during balloon expansion. Since then, PLLA have been employed by companies such as Abbott Vascular to allow expansion of bioresorbable stents at body temperature. In addition, companies like Cordis, Biosensors, Tepha and Orbus Neich have used PLLA to allow stent expansion at body temperature2. Non-lactide based polymers such as tyrosine-derived polycarbonate stents have also been developed for use in bioabsorbable stents from the collaboration of Rutgers University (Rutgers, NJ, USA) and REVA Medical (San Diego, CA, USA).
Elixir Medical bioresorbable drug eluting stent (BDES)
The bioresorbable stent of Elixir Medical Corporation (Sunnyvale, CA, USA) is developed to address the needs of balancing the competing requirements of the temporary implant to provide a “no compromise” system.
Stent material
Elixir utilises PLLA as its base polymer material. PLLA has an extensive history of use in medical applications including uses in orthopaedics, cardiology and medical sutures. Elixir is extensively evaluating means to control polymer material properties such as strength, recoil and degradation profile as well as using proprietary fabrication and processing technologies to enable expansion of the stent at physiological temperature with minimal recoil and optimised radial strength. Based on these processes, the Elixir BDES stent is expected to biodegrade and resorb over a period of 2-3 years. The resorption of these polymers occurs mainly by hydrolysis and begins with loss in molecular weight or decreases in the number of repeating monomer units followed by loss in strength and mass. This produces by-products which are metabolised into pyruvic acid required for the Krebs Cycle. It is ultimately excreted from the body as carbon dioxide and water3.
Pharmaceutical agent
Elixir Medical has multiple pharmaceutical agents in its drug product portfolio. Elixir developed Novolimus™, a novel mTOR inhibitor macrocyclic lactone, with antiproliferative and anti-inflammatory properties, for drug eluting stent applications. Elixir has an exclusive worldwide license for use of Myolimus in fully biodegradable stents. Myolimus™ is a novel mTOR inhibitor macrocyclic lactone with a broad therapeutic index and an excellent stability profile which makes it desirable for BDES applications.
BDES stent platform & delivery system
Multiple stent designs have been evaluated in the Elixir BDES program, including the samples depicted in Figure 1. Elixir’s rapid exchange delivery system is composed of a stainless steel hypotube proximal shaft, nylon blend distal shaft, and nylon blend balloon material. Figure 2 shows a sample BDES stent on a stent delivery system both in the crimped and expanded states.

Figure 1. Sample stent designs with radioapaque markers (centre) evaluated in the Elixir BDES program.

Figure 2. Sample stent pre-mounted on a 3.0mm delivery system in crimped and deployed states.
A summary of the stent technical specifications is provided below:
Material composition: PLLA-based polymer
Radiopaque markers: Markers at proximal and distal ends
Degree of radiopacity: Moderate with markers
Ferro-magnetism: Non-ferromagnetic (MRI compatible)
Deployment mechanism: Balloon expandable
Minimum internal diameter
of guiding catheter: 0.075 inch (1.9 mm), 7 Fr
BDES radial strength
Compressive radial strength of sterilised stents was measured using a low friction iris-type radial compression tester connected to a tensile tester. The devices were exposed to a 37°C circulating water bath, expanded to the intended diameter and then inserted into the radial compression fixture which was also in a 37°C water bath; a circumferential compressive force was then applied. The radial compression fixture was actuated at a controlled rate by a tensile test stand such that the force vs diameter change were measured, and a force-displacement curve generated. The force-displacement curve was used to calculate the stent stiffness (modulus) and the force required to resist 15% diameter compression. Compressive radial strength for different stent designs as compared to Elixir’s bare metal alloy stent are presented in Figure 3.
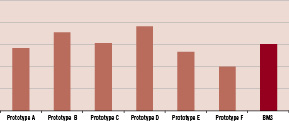
Figure 3. Compressive radial strength of Elixir BDES prototypes with different design and processing parameters compared to Elixir’s bare metal cobalt-chromium alloy stent (BMS).
BDES recoil
Similarly, recoil testing of sterilised stents was conducted by expanding the stent to nominal diameter in a 37°C circulating water bath. The stent diameter was measured using an optical comparator and inspected for evenness of expansion and stent integrity. The balloon was then deflated and the stent maintained in the water bath for one hour before the diameter was re-measured. Changes in diameter were noted, and the amount of recoil was calculated based on initial diameter vs final diameter at one hour. The change in expanded diameter recorded as percentage recoil for different stent designs as compared to Elixir’s bare metal alloy stent are presented in Figure 4.
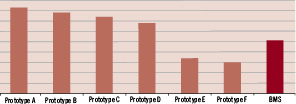
Figure 4. Percentage recoil of Elixir BDES prototypes with different design and processing parameters compared to Elixir’s bare metal cobalt-chromium alloy stent (BMS).
The radial strength and recoil data suggests that stent design and material processing parameters play a significant role in the mechanical performance of the BDES stent, and in fact, the mechanical properties of the BDES can be comparable to bare metal stents.
Elixir has initiated preclinical testing of the polymer only resorbable stent and multiple BDES prototypes in the porcine coronary artery model to evaluate the biological response to stent implantation over different periods of time. Additional drug pharmacokinetic studies and bioresorption studies of the stent material are also ongoing.
Conclusion
Elixir Medical is developing a PLLA based fully bioresorbable stent with mechanical properties and delivery system performance similar to currently available metallic stent platforms. Preclinical studies are ongoing to evaluate the biological response, pharmacokinetics and bioresorption profile of the stent in a porcine coronary artery model. Upon successful completion of these studies, Elixir will evaluate the BDES in a FIM clinical trial.

