Background
Myocardial infarctions are typically the result of the disruption of complex thin-cap fibroatheromas (TCFA)1,2 and it is quite reasonable that many have suggested that these lesions react similarly to the implantation of devices currently employed for symptomatic obstructive disease3. Balloon-expandable scaffoldings are the real workhorses of the trade, providing succesfully high dilating forces for almost any type of obstructive lesions. In-stent restenosis following stent implantation has been succesfully managed by the controlled release of anti proliferative drug coatings applied directly to the stent surface4. Unfortunately, recent clinical evidence suggest the potential for thrombogenicity and delayed vascular healing after device implantation5,6. Therefore, in their current form, balloon-expandable scaffoldings do not appear to be a proper approach to pre-emptive treatment of high risk plaques. While high radial forces may be needed to dilate harder, obstructive lesions, they impose more injury than may be necessary in softer, less occlussive lesions. Despite the fact self-expanding stents (SE) dominate the peripheral market, they have found little acceptance in the coronary market due to a combination of bulky delivery systems, poor deployment accuracy, migration and concerns about chronic over-expansion yielding progressive vessel injury7. However, due to its intrinsic mechanical properties, self expandable scaffoldings could become the ideal vascular prosthesis to use in non-obstructive and relatively soft lesions.
Device description
The vProtect™ luminal shield system consists of the self expanding vascular shield and a rapid exchange delivery system. The delivery system is compatible with .014” guidewires and 6 Fr Guiding catheters. The usable length is 135 cm with a rapid exchange guidewire lumen of 25 cm. The delivery systems consists of a distal outer sheath that houses the luminal shield and an inner body with radiopaque markers at the distal and proximal ends of the Shield. The luminal shield is constructed from a nickel-titanium alloy with an austenetic finish (Af) temperature between 20 and 25 degrees centigrade (temperature at which the device achieves its full radial force). The shield has a wall thickness that is less than 70 microns and has been designed with the objective to match the elastic properties of the TCFA (Figure 1).
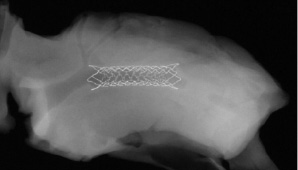
Figure 1. Representative shield design implanted in a swine coronary artery. Note both flared ends designed for mechanical stabilisation of the shoulder of the plaque. Image courtesy of PMI.
The shield has a percent open area ranging from 13% to 16% in 2.5 to 3.0 mm vessels.
Indications for use
Although the development of this device is still in evolution, its intended indication for use is the mechanical stabilisation of non-obstructive, soft lesions in the coronary territory. However, in the clinical practice and due to the mechanical properties of the device, the vascular shield might achieve the following objectives:
– Treat non-obstructive, non-calcified lesions (moderate plaque burden)
– Treat obstructive, but soft lesions in the right clinical setting
– Treat lesions located at bifurcation points
– Induce minimal injury to the vessel wall
– “Reshape” the lumen and “reinforce” the fibrous cap
– “Remodel” the necrotic core
– Induce minimal neointimal formation and promote endothelialisation
Also, potential contraindications for the use of this device could include:
– Lesions with a very high plaque burden
– Very calcified lesions in which high radial strength is needed
– Very long / very tortuous lesions
Pre-clinical studies
Preliminary studies using the rabbit balloon denudation model were designed to determine the impact of radial force on vascular injury and healing. Two different studies have been completed using the rabbit iliac injury model at two different time points (seven and 14 days). These studies utilised three different shield designs (16.9 mm length) with different geometrical designs, radial forces and mechanical properties. After selection of one of the shield designs, an acute study using three normal pigs was performed using three coronary arteries to test the shield capabilities of anchoring to the vessel wall without migration. Comparative studies using the Vision™ and the Xience™ as stent controls are under development using the coronary porcine model.
Experimental methodology
In the rabbit studies, the animals were anesthetised by ketamine (35 mg/kg) and xylazine (5 mg/kg) and maintained on isofluorane. The iliac arteries were injured twice by endothelial denudation prior to stent delivery. A 3.0x10 mm balloon catheter was placed in the distal iliac artery, using standard fluoroscopy methods, and was inflated to 6 atm (approximate balloon to artery ratio=1.3:1). The catheter was then withdrawn proximally in its inflated state at a distance of approximately 1 cm. Bilateral shields implants were deployed in the iliofemoral vessels of each rabbit with the pre-mounted delivery system delivered to each iliac artery over a guidewire using fluoroscopic guidance. In the acute and 28 days porcine studies, carotid access was used and angiography and IVUS/OCT were used at baseline and at termination. In the acute study, OCT images (LightLab) were acquired using saline flush and vessel and shields areas where analysed for strut malapposition. At completion of the study, the vessels were harvested and fixed with continued gravity perfusion with 10% neutral-buffered formalin. All the shields were carefully removed and immersions fixed overnight and the specimens were hand carried to CVPath, Gaithersburg Md, USA, for histomorphometric analysis. Sections from the shields were cut on a rotary microtome at four to six microns, mounted and stained with hematoxylin and eosin and elastic Van Gieson stains. All sections were examined by light microscopy for the presence of inflammation, thrombus, neointimal formation and vessel wall injury.
Results
In the first rabbit study, a total of 24 vascular shields (design A= 8, design B=9, design C=7) were implanted in 24 iliacs. Animals were sacrificed at seven days for histological evaluation. In this study, the mean injury scores were particularly low (design A=0.13±0.026, design B=0.044±0.088, design C=0.067±0.071). Lumen areas varied according to the radial force of the device; design A= 6.32±0.36 mm2, design B=4.43±0.38 mm2 and design C= 5.13±0.87 mm2. Malapossition of the device was not found on histology. At seven days, endothelialisation above the struts varied from 39.0±12.8% in design B to 51.7±35.4% in design A (Figure 2).
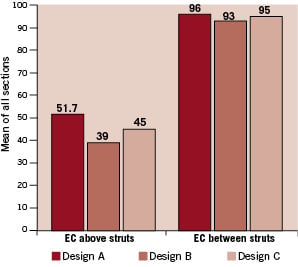
Figure 2. Assessment of device endothelialisation at 7 and 14 days after shield implantation in the rabbit iliac denudation model.
Near complete device endothelialisation (>90%) above the struts was noted in all devices at 14 days (Figure 3).
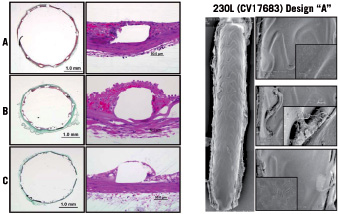
Figure 3. Assessment of device endothelialisation at 14 days after shield implantation in the rabbit iliac denudation model. Images courtesy of PMI.
Acute studies performed in normal coronary arteries in the pig have also been completed using a single modified shield design derived from the rabbit studies. In these acute experiments, the vascular shield was able to anchor to the target vessel segment without any distal migration induced by the blood flow. Shield evaluation performed by OCT demonstrated complete wall apposition and device conformability to the vessel morphology and diameters (Figure 4).
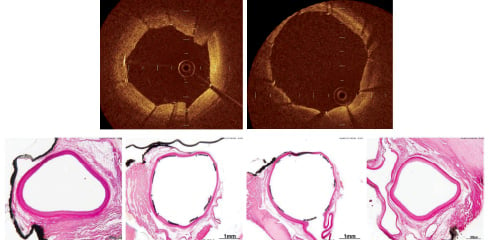
Figure 4. Acute implants in porcine coronary arteries. Upper panel shows device apposition in vivo using OCT. Lower panel shows histological appearance of the device right after implantation. Images courtesy of PMI.
A 28 day study has been recently completed using the porcine coronary artery restenosis model (1:1.1 overstretch ratio). In this study, 10 vascular shields were implanted in 10 arteries and compared to the Vision stent (n=10) and the Xience stent (n=10). In this study, at 28 days the angiographic restenosis was 20.70%±9.83% in the shield, 25.50%±16.96% in the Vision stent and 17.40%±12.54% in the Xience stent group P=N.S). Histological data is under analysis. Overall the injury score in the shield was lower than in both balloon expandable devices and the restenosis rates comparable in all three groups (area of stenosis; shield 27.6%±9.04%, Vision 28.47%±10.94%, Xience stent 21.21%±7.66%, p=N.S).
Conclusions
Current practices and available technologies in focal treatment are primarily focused on improving luminal diameter in occlusive plaques and are not well developed for the treatment of the TCFA. More focus is needed on achieving the main objectives of focal therapy; a) mechanical stabilisation, b) promotion of vascular healing and c) reduction of inflammation. The vProtect™ luminal shield system was designed with the objective of finding a point of equilibrium between the device’s intrinsic expansive force and resistive mechanical properties of the TCFA. Preliminary pre-clinical data suggests that despite its relatively low radial force, this device anchors properly to the vessel wall, maintains its mechanical structure up to 28 days and promotes healthy endothelialisation in rabbit iliac arteries. The potential technical advantages of this device such as reinforcement of the fibrous cap and “re-shaping” of the necrotic core leading to mechanical stabilisation of the TCFA needs to be studied in diseased animal models and tested in the proper clinical setting. In addition, the regeneration of the endothelium and recovery of its functionality by means of passive or active endothelial cell attraction may enhance the potential of this device to achieve proper vascular healing in the setting of focal therapy of TCFA.
Online data supplement
Supplementary data
To read the full content of this article, please download the PDF.
Vidéo 1

